
Exploring the psychological profile: a cross-sectional study of 1,185 patients with pulmonary nodules in an outpatient clinic
Introduction
Lung cancer is one of the leading causes of cancer-related death worldwide (1). During the last decade, efforts to increase the rate of early detection using low-dose computed tomography (CT) screening have led to the increased discovery of indeterminate pulmonary nodules (IPNs). Pulmonary nodules (PNs) are defined as abnormal opacities smaller than 3 cm in the lung parenchyma (2). Many patients consider PNs to be precancerous lesions or even early-stage lung cancer although most nodules are benign in nature (3). A rigorous follow-up plan and active surveillance, which may last for years, are usually recommended for nodules with indeterminate biological behaviors (2). This “watch and wait” approach, along with the fear of getting cancer, inevitably increases the psychological burden on these patients (4).
Recently, individuals with PNs have been found to be vulnerable to emotional distress or psychological burden (5). Studies among patients with PNs have shown that sex, age, education level, and smoking history may be the positive predictors of nodule-related distress (6-8), while high-quality patient-clinician communication, social support, and noninvasive methods, such as a PN fact sheet, are associated with decreased distress (9-11). However, the psychological burden as well as the values and preferences of patients in the thoracic outpatient clinic have not been given much attention due to the large number of patients with PNs, which limits the duration of consultations. Interestingly, a recent study found that anxious patients were more likely to adopt aggressive management, leading to higher rates of false-positive misdiagnoses and unnecessary surgeries (4). In fact, it has been reported that a high proportion of patients with PNs who underwent surgery had benign diseases (12,13). This undoubtedly increases the burden on the health care system and results in wastage of medical resources. Disease-related psychological burden is a psychosocial condition, which is heavily influenced by personal and environmental or social factors, some of which may be modifiable through proper health education or patient-centered communication. Thus, there is a need to identify potential risks or protective factors for disease-related psychological burden in a large sample. The data generated may contribute to the prevention of overtreatment of patients with PNs and preserve medical resources for others in need (14).
Management of PNs varies from one clinician to another due to the lack of a standard approach based on current evidence, especially for those with nodules of an indeterminate or indolent nature. Although medical recommendations are usually based on clinical guidelines, the patients’ overwhelming anxiety levels sometimes cause clinicians to deviate from such recommendations (15). Since psychosocial issues have remained underrecognized and undertreated for a long period of time, emerging guidelines also emphasize the importance of eliciting patients’ treatment preferences (16). In particular, communication processes that evaluate patients’ preferences and values have been associated with decreased distress and with helping patients to make sound decisions (6,17). However, patients’ extrinsic and intrinsic motivations to undergo treatment options that are not evidence-based are not completely understood. We hypothesized that there may be risk factors for elevated levels of psychological burden and that knowledge of such factors may assist with the prompt identification of patient groups that need psychological consultation or tailored health education.
In this cross-sectional study, we carried out a questionnaire-based survey among patients with PNs in an outpatient clinic to identify the potential risk or protective factors for psychological burden. One aim of the study is to help with better understanding of how patients’ psychological status affects their treatment preferences. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-847/rc).
Methods
Participants
This cross-sectional study was conducted in the Thoracic Clinic of Guangdong Provincial People’s Hospital from January 1, 2021, to November 30, 2021. Patients with PNs who attended the outpatient clinic for the first time were invited to complete self-administered questionnaires. The inclusion criteria were as follows: (I) diagnosed with solitary or multiple IPNs smaller than 3 cm on a high-resolution CT scan; (II) aged 18 years and over; and (III) the ability to complete the questionnaires and sign the informed consent form. The exclusion criteria were as follows: (I) known pathological diagnosis of PNs by biopsy or surgery; (II) previously diagnosed mental disorder; and (III) previous history of malignancy at any site of the body.
Data collection
The research protocol and data collection forms were predefined and reviewed by a multidisciplinary expert panel formed by surgeons, public health specialists, statisticians, and patient representatives. Demographic and clinical data were collected using a self-administered questionnaire, which included age, gender, smoking status, family history of malignancy, symptomatic complaints, reasons for CT workup, number and size of PNs, and dynamic change of PN during follow-up. Communication factors, including adherence to doctors’ recommendation and perception of follow-up periods recommended by doctors, were also assessed. The evaluation of psychological burden was undertaken using the Chinese version of the Hospital Anxiety and Depression Scale (HADS) (18,19).
The HADS consists of two subscales to assess anxiety and depression and is a reliable self-reporting tool, which is commonly used in clinical practice. Each subscale consists of seven 4-point Likert scale items, with the total score ranging from 0 to 21, whereby a higher score indicates higher levels of anxiety or depression. Based on the validation of the questionnaire, scores between 0 and 7 are considered within the normal range, while scores ≥8 suggest anxiety and depression (20).
Data collection was primarily conducted via web-based administration of the questionnaire using Wen Juan Xing (https://www.wjx.cn/; Changsha, China), the largest online platform for design and delivery of surveys in China. Patients who did not have access to the internet completed their questionnaires using available tablets and paper or through oral survey.
Statistical analysis
Demographic and clinical characteristics are described using mean with standard deviation (SD) for continuous data and numbers (percentages) for categorical data. The Shapiro-Wilk test was used to determine if the data were normally distributed. Categorical variables were compared using the Pearson chi-squared test, while continuous variables were compared using the Kruskal-Wallis test. Binary logistic regression analysis was used to assess independent associations and their respective odds ratio (OR) regarding depression and anxiety. All tests were two-sided, and a P value <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS (version 26.0., IBM Corp, Armonk, NY, USA) software. A heatmap was generated using the ComplexHeatmap package in R v. 3.4.422 (The R Foundation for Statistical Computing), while a violin plot and a bar plot were generated using GraphPad Prism Version 8 (GraphPad Software, San Diego, CA, USA).
Ethical statement
This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. KY-Q-2021-005-03) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All the patients enrolled in this study provided informed consent. This study is part of a study registered in ClinicalTrials.gov (registration No. NCT04857333).
Results
Demographic and clinical characteristics
Of the 1,284 patients invited for this psychological screening study, 1,185 (92.3%) provided informed consent and completed both the baseline information collection form and the psychological inventory. The demographic and clinical characteristics of the participants are summarized in Table 1, with their detailed information and corresponding psychological profile depicted using a comprehensive heatmap (Figure 1). In brief, the mean age of the overall cohort was 47 years (SD =11.4), and most of them (87.0%) were younger than 60. Females accounted for 61.8% of all patients with PNs. Notably, 77.4% of the patients with PNs were never-smokers, while only a small proportion of patients (7.5%) were current smokers, and the remainder had quit smoking. Family history of malignancy, which was defined as any malignant neoplasm in direct blood relatives within 3 generations, was documented in 53.8% of patients. Interestingly, 60.7% of the patients with PNs reported one or more symptoms, such as abnormal sensation of the throat (n=382), cough (n=291), chest pain (n=176), expectoration (n=168), and hemoptysis (n=15). The proportions of patients with solitary or multiple PNs were balanced, at 48.5% vs. 51.5% of patients, respectively. Primary lesions with a maximum diameter of ≤10, 11 to 20, and 21 to 30 mm accounted for 81.7%, 16.2%, and 2.1% of the study cohort, respectively. In terms of the biological behavior, 76.3% of the PNs were indeterminate, while only 16.4% of nodules were deemed benign according to the initial impression of the doctors involved. The results also showed that 1,141 (96.3%) patients reported they would follow the doctor’s recommendation for follow-up periods, while 1,018 (85.9%) thought the timing of follow-up was appropriate.
Table 1
| Variables | Overall cohort, n (%) | HADS-anxiety | HADS-depression | |||
|---|---|---|---|---|---|---|
| N (%) | P value† | N (%) | P value† | |||
| Age (years) | 0.054 | 0.556 | ||||
| <35 | 202 (17.0) | 91 (45.0) | 60 (29.7) | |||
| 35–59 | 830 (70.0) | 366 (44.1) | 222 (26.7) | |||
| ≥60 | 153 (13.0) | 52 (34.0) | 38 (24.8) | |||
| Gender | 0.151 | 0.680 | ||||
| Male | 453 (38.2) | 198 (43.7) | 133 (29.4) | |||
| Female | 732 (61.8) | 311 (42.5) | 187 (25.5) | |||
| Smoking status | 0.389 | 0.314 | ||||
| Current | 89 (7.5) | 44 (49.4) | 26 (29.2) | |||
| Ever | 179 (15.1) | 71 (39.7) | 55 (30.7) | |||
| Never | 917 (77.4) | 394 (43.0) | 239 (26.1) | |||
| Family history of malignancy | 0.023* | 0.149 | ||||
| Yes | 637 (53.8) | 293 (46.0) | 183 (28.7) | |||
| No | 548 (46.2) | 216 (39.4) | 137 (25.0) | |||
| Subjective symptoms | <0.001* | <0.001* | ||||
| Yes | 719 (60.7) | 349 (48.5) | 224 (31.2) | |||
| No | 466 (39.3) | 160 (34.3) | 96 (20.6) | |||
| Reasons for CT workup | 0.001* | 0.029* | ||||
| Physical discomfort | 230 (19.4) | 124 (53.9) | 78 (33.9) | |||
| Health check-up | 373 (31.5) | 152 (40.8) | 92 (24.7) | |||
| Follow-up exam for PN | 582 (49.1) | 233 (40.0) | 150 (25.8) | |||
| PN number | <0.001* | <0.001* | ||||
| Solitary | 575 (48.5) | 214 (37.2) | 128 (22.3) | |||
| Multiple | 610 (51.5) | 295 (48.4) | 192 (31.5) | |||
| Size of PN‡ | 0.873 | 0.570 | ||||
| ≤10 mm | 968 (81.7) | 415 (42.8) | 261 (36.0) | |||
| 11 to 20 mm | 192(16.2) | 82 (42.7) | 50 (26.0) | |||
| 21 to 30 mm | 25(2.1) | 12 (48.0) | 9 (26.9) | |||
| Change during follow-up | 0.810 | 0.965 | ||||
| Initial diagnosis | 328 (27.7) | 142 (43.3) | 91 (27.9) | |||
| No obvious change | 552 (46.6) | 230 (41.7) | 146 (26.4) | |||
| Progression | 281 (23.7) | 127 (45.2) | 77 (27.2) | |||
| Remission | 24 (2.0) | 10 (41.7) | 6 (25.0) | |||
| Initial impression of doctors | 0.273 | 0.050 | ||||
| Indeterminate | 904 (76.3) | 394 (43.6) | 249 (27.5) | |||
| Benign | 194 (16.4) | 74 (38.1) | 41 (21.1) | |||
| Malignant | 87 (7.3) | 41 (47.1) | 30 (34.5) | |||
| Adherence to doctors’ recommendation | 0.733 | 0.967 | ||||
| High adherence | 1,141 (96.3) | 489 (42.9) | 308 (27.0) | |||
| Low adherence | 44 (3.7) | 20 (45.5) | 12 (27.3) | |||
| Perception of follow-up periods recommended by doctors | <0.001* | <0.001* | ||||
| Appropriate | 1,018 (85.9) | 407 (40.0) | 243 (23.9) | |||
| Too long | 119 (10.0) | 79 (66.4) | 58 (48.7) | |||
| Too short | 48 (4.1) | 23 (47.9) | 19 (39.6) | |||
†, chi-square test; *, statistical significance; ‡, diameter of primary lesion in patients with multiple nodules. PN, pulmonary nodule; HADS, Hospital Anxiety and Depression Scale; CT, computed tomography.
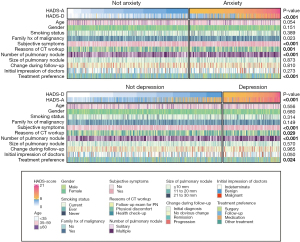
Disease-related psychological profile
As is shown in Figure 1, patients with PNs were more vulnerable to anxiety than to depression. A higher proportion of participants tested positive (scored ≥8) for abnormal psychological status under the HADS-anxiety subscale (n=509, 42.1%) compared with the HADS-depression subscale (n=320, 27.0%), with the mean scores being 11.49 (SD =3.29) and 11.13 (SD =3.01), respectively. The demographic and clinical characteristics of these anxious or depressed patients are presented in Table 1. Patients with a family history of malignancy were significantly more likely to test positive for anxiety than were those without a family history of malignancy [46.0% (293/637) vs. 39.4% (216/548); χ2=5.206; P=0.023]. Patients who had subjective symptoms were significantly more likely to test positive for anxiety [48.5% (349/719) vs. 34.3% (160/466); χ2=23.283; P<0.001] and depression [31.2% (224/719) vs. 20.6% (160/466); χ2=15.976; P<0.001]. Similarly, patients who had CT scans due to physical discomfort were more likely to be anxious [53.9% (124/230) vs. 40.3% (385/955); χ2=14.037; P=0.001] and depressed [33.9% (78/230) vs. 25.3 (242/955); χ2=7.052; P=0.029] than were those who did not feel uncomfortable. It is worth noting that significantly higher rates of both anxiety and depression were found in patients with multiple nodules (anxiety: χ2=14.999, P<0.001; depression: χ2=14.999, P<0.001) in patients who perceive follow-up periods were too long (anxiety: χ2=30.822, P<0.001; depression: χ2=37.445, P<0.001). However, univariate analysis showed that age, gender, smoking status, size of PNs, change of nodule during follow-up, and adherence to doctors’ recommendation were not correlated with anxiety and depression (all P values >0.05).
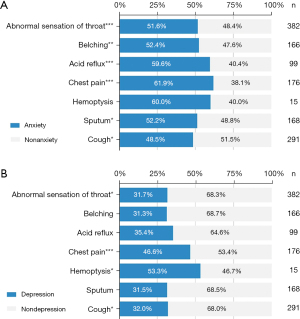
It is worth noting that subjective symptoms strongly affected the psychological burden. Thus, as demonstrated in Figure 2, we further explored the relationship between patients’ specific symptoms and psychological burden. There was a significantly higher positive rate of anxiety in patients with symptoms including abnormal sensation of throat (P<0.001), belching (P<0.01), acid reflux (P<0.001), chest pain (P<0.001), sputum (P<0.05), and cough (P<0.05). There was also a significantly higher positive rate of depression in patients with symptoms, such as abnormal sensation of throat (P<0.05), chest pain (P<0.001), hemoptysis (P<0.05), and cough (P<0.05).
To identify the variables that were independently associated with anxiety and depression, we conducted a binary logistic regression analysis (Table 2). The results indicated that age, reason for CT workup, number of PNs, family history of malignancy, and subjective symptoms were predictors of anxiety, while number of PNs, subjective symptoms, and initial impression of doctors predicted depression. Patients aged 60 years or more had lower odds for anxiety, compared to those aged <35 (OR =0.57; 95% CI: 0.36–0.89; P=0.014). In contrast to those who did not undergo CT, patients who underwent a CT scan due to physical discomfort were more likely to be anxious (OR =1.58; 95% CI: 1.11–2.24; P=0.008). In addition, multiple PNs (OR =1.52; 95% CI: 1.20–1.94; P=0.001), family history of malignancy (OR =1.28; 95% CI: 1.01–1.64; P=0.044), and subjective symptoms (OR =1.70; 95% CI: 1.32–2.19; P<0.001) were independently associated with anxiety. Meanwhile, number of PNs (OR =1.51; 95% CI: 1.15–1.98; P=0.003) and subjective symptoms (OR =1.65; 95% CI: 1.23–2.20; P=0.001) were also predictors of depression. Moreover, given the initial impression of doctors, patients were more anxious when the nature of the PNs was perceived to be indeterminate than when the PNs were considered to be benign (OR =1.91; 95% CI: 1.08–3.40; P=0.027). After communication with their doctors, patients who felt follow-up periods recommended by doctors were appropriate were less likely to be anxious and depressed (anxiety: OR =0.37, 95% CI: 0.25–0.56, P<0.001; depression: OR =0.36, 95% CI: 0.24–0.53, P<0.001) compared with those who felt that the recommended follow-up periods were too long.
Table 2
| Variables | HADS-anxiety | HADS-depression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value† | OR (95% CI) | P value† | ||
| Age (years) | |||||
| <35 | 1 | ||||
| 35–59 | 0.95 (0.69–1.30) | 0.733 | |||
| ≥60 | 0.57 (0.36–0.89) | 0.014 | |||
| Reasons for CT workup | |||||
| Health check-up | 1 | 1 | |||
| Follow-up exam for PN | 0.94 (0.71–1.23) | 0.635 | 1.03 (0.75–1.40) | 0.866 | |
| Physical discomfort | 1.58 (1.11–2.24) | 0.008* | 1.42 (0.97–2.08) | 0.071 | |
| PN number | |||||
| Solitary | 1 | 1 | |||
| Multiple | 1.52 (1.20–1.94) | 0.001* | 1.51 (1.15–1.98) | 0.003* | |
| Family history of malignancy | |||||
| No | 1 | 1 | |||
| Yes | 1.28 (1.01–1.64) | 0.044* | 1.17 (0.89–1.53) | 0.267 | |
| Subjective symptoms | |||||
| No | 1 | 1 | |||
| Yes | 1.70 (1.32–2.19) | <0.001* | 1.65 (1.23–2.20) | 0.001* | |
| Initial impression of doctors | |||||
| Benign | 1 | ||||
| Malignant | 1.30 (0.89–1.91) | 0.202 | |||
| Indeterminate | 1.91 (1.08–3.40) | 0.027* | |||
| Perception of follow-up periods recommended by doctors | |||||
| Too long | 1 | 1 | |||
| Too short | 0.55 (0.27–1.10) | 0.090 | 0.79 (0.40–1.59) | 0.510 | |
| Appropriate | 0.37 (0.25–0.56) | <0.001* | 0.36 (0.24–0.53) | <0.001* | |
†, chi-square test; *, statistically significance. PN, pulmonary nodule; HADS, Hospital Anxiety and Depression Scale; OR, odds ratio; CI, confidence interval; CT, computed tomography.
Impact on the treatment preferences of PNs
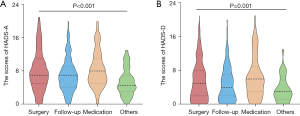
All the patients answered the item regarding what treatment options they would prefer if the nodule was clinically characterized as suspicious for malignancy. As demonstrated in Table 3 and illustrated in Figure 3, patients who screened positive for anxiety and depression chose more aggressive managements compared with patients who had a negative screen for anxiety and depression (P<0.001 and P=0.001, respectively). Specifically, the proportion of patients choosing surgery in patients who were positive for anxiety and depression was significantly higher than that in patients who were negative (42.6% vs. 34% and 43.1% vs. 35.7%, respectively). In contrast, patients who were negative for anxiety and depression were more willing to consent to follow-up compared to those who were positive (55.0% vs. 45.8% and 53.2% vs. 45.3%, respectively). It is worth noting that subjective symptoms significantly influenced psychological burden, which in turn had an impact on treatment preferences (χ2=9.696; P=0.021). Moreover, the change of PNs during follow-up also had an important effect on treatment preferences (χ2=18.198; P=0.033). As for communication factors, patients who thought the follow-up periods recommended by doctors were too long were more likely to prefer surgery (χ2=18.198; P<0.001).
Table 3
| Variables | Treatment preferences, n (%) | P value† | |||
|---|---|---|---|---|---|
| Surgery | Follow-up | Medication | Other treatment | ||
| Age (years) | 0.189 | ||||
| <35 | 68 (33.7) | 114 (56.4) | 14 (6.9) | 6 (3.0) | |
| 35–59 | 329 (39.6) | 413 (49.8) | 68 (8.2) | 20 (2.4) | |
| ≥60 | 50 (32.7) | 78 (51.0) | 19 (12.4) | 6 (3.9) | |
| Gender | 0.788 | ||||
| Male | 169 (37.3) | 230 (50.8) | 43 (9.5) | 11 (2.4) | |
| Female | 278 (38.0) | 375 (51.2) | 58 (7.9) | 21 (2.9) | |
| Smoking status | 0.750 | ||||
| Current | 31 (34.8) | 47 (52.8) | 8 (9.0) | 3 (3.4) | |
| Ever | 64 (35.8) | 89 (49.7) | 21 (11.7) | 5 (2.8) | |
| Never | 352 (38.4) | 469 (51.1) | 72 (7.9) | 24 (2.6) | |
| Family history of malignancy | 0.148 | ||||
| Yes | 249 (39.1) | 321 (50.4) | 46 (7.2) | 21 (3.3) | |
| No | 176 (36.7) | 254 (53.0) | 41 (8.6) | 8 (1.7) | |
| Subjective symptoms | 0.021* | ||||
| Yes | 274 (38.1) | 351 (48.8) | 75 (10.4) | 19 (2.6) | |
| No | 173 (37.1) | 254 (54.5) | 26 (5.6) | 13 (2.8) | |
| Reasons for CT workup | 0.437 | ||||
| Physical discomfort | 91 (39.6) | 107 (46.5) | 24 (10.4) | 8 (3.5) | |
| Health check-up | 149 (39.9) | 184 (49.3) | 32 (8.6) | 8 (2.1) | |
| Follow-up exam for PN | 207 (35.6) | 314 (54.0) | 45 (7.7) | 16 (2.7) | |
| PN number | 0.528 | ||||
| Solitary | 226 (39.3) | 291 (50.6) | 45 (7.8) | 13 (2.3) | |
| Multiple | 221 (36.2) | 314 (51.5) | 56 (9.2) | 19 (3.1) | |
| Size of PN‡ | |||||
| ≤10 mm | 357 (36.9) | 507 (52.4) | 76 (7.9) | 28 (2.9) | 0.057 |
| 11 to 20 mm | 81 (42.2) | 88 (45.8) | 19 (9.9) | 4 (2.1) | |
| 21 to 30 mm | 9 (36.0) | 10 (40.0) | 6 (24.0) | 0 (0.0) | |
| Change during follow-up | 0.033* | ||||
| Initial diagnosis | 144 (43.9) | 142 (43.3) | 32 (9.8) | 10 (3.0) | |
| No obvious change | 182 (33.0) | 314 (56.9) | 41 (7.4) | 15 (2.7) | |
| Progression | 114 (40.6) | 135 (48.0) | 26 (9.3) | 6 (2.1) | |
| Remission | 7 (29.2) | 14 (58.3) | 2 (8.3) | 1 (4.2) | |
| Initial impression of doctors | 0.380 | ||||
| Indeterminate | 348 (38.5) | 452 (50.0) | 80 (8.8) | 24 (2.7) | |
| Benign | 66 (34.0) | 104 (53.6) | 19 (9.8) | 5 (2.6) | |
| Malignant | 33 (38.0) | 49 (56.3) | 2 (2.3) | 3 (3.4) | |
| Adherence to doctors’ recommendation | 0.158 | ||||
| High adherence | 430 (37.7) | 587 (51.4) | 95 (8.3) | 29 (2.5) | |
| Low adherence | 17 (38.6) | 18 (40.9) | 6 (13.6) | 3 (6.8) | |
| Perception of follow-up periods recommended by doctors | <0.001* | ||||
| Too long | 67 (56.3) | 36 (30.3) | 11 (9.2) | 5 (4.2) | |
| Too short | 12 (25.0) | 26 (54.3) | 8 (16.7) | 2 (4.2) | |
| Appropriate | 368 (36.1) | 543 (53.2) | 82 (8.1) | 25 (2.5) | |
†, chi-square test; *, statistically significance; ‡, the diameter of primary lesion in patients with multiple nodules. PN, pulmonary nodule; CT, computed tomography.
Discussion
PNs have become increasingly prevalent and represent a medico-societal concern. Currently, the dominant management strategy for most PNs is active surveillance based on nodule characteristics and their corresponding risk levels (2,21-23). During active surveillance, most patients suffer from psychological distress (24), which may contribute to more aggressive treatment decisions, impact on doctors’ judgments and decisions (15,25), and pose a considerable challenge for the management of PNs. Even though we investigated the factors associated with psychological distress in patients with PNs, an even larger sample size may be needed to assess the potential factors from multiple dimensions. The results presented may allow for the prompt identification of distressed patients in the clinical setting and lead to the better management of PNs.
Our findings corroborate the impact of PN detection on emotional health demonstrated in prior studies (5,9,25). Psychological distress is commonly observed among patients with PNs, who show higher anxiety and depression scores compared with the normal population (26,27). Notably, there was a significant correlation between anxiety and depression in our study: while anxiety occurred more frequently, a large proportion of patients with depression also had anxiety. These psychological changes may be ascribed to a fear of developing cancer and insufficient knowledge about the disease. In psychology, anxiety is the body’s defensive response when coping with interior or exterior stresses (28), while depression usually correlates with behaviors related to withdrawal (29). A defensive response is usually triggered initially after the accidental detection of PNs. When fear and anxiety persist, the psychological burden is exacerbated and contributes to the development of depression. It has been reported that anxiety and depression are bidirectional risk factors for each other, and thus the presence of depression and anxiety complicates patient management (30,31). Unfortunately, our study showed that patients who had both anxiety and depression accounted for a considerable proportion. Therefore, earlier identification and timely psychological intervention are of great significance for these patients.
Concerning the possible risk factors of anxiety and depression in patients with PNs, in our study patients <35 years old were more likely to be anxious, which is consistent with reports that younger age is a predictor for anxiety for patients with PNs and other acute or chronic diseases, such as cancer and glaucoma (8,32,33). However, PNs in young people are more likely to be caused by infection, and as such young patients’ anxiety appears excessive. In our study, patients with a family history of malignancy also had a higher proportion HADS-anxiety scores, which is consistent with the findings by Lee et al. (34), who reported that patients with a family history of malignancy were more anxious during breast cancer screening. The results of our study also revealed that the presence of indeterminate nodules was associated with depression in patients with PNs. As shown in Table 1, IPNs accounted for 76.3% of all nodules and caused confusion and excessive worry in patients due to misperceptions about the natural history of nodules (6). Remarkably, the symptoms and number of PNs were significantly positive predictors of both anxiety and depression. It can be speculated that the number of PNs may be considered a sign of whether the PNs have metastasized. Therefore, patients should be educated about nodules to avoid excessive psychological burden. Strategies identified by other studies, such as a brochure that explained the safety and favorable outcomes for most nodules (10) and tailoring patient-centered communication (15), may be helpful.
Symptoms are often the most common cause of physical discomfort for patients and the main reason for visiting a doctor. In the current study, there was a strong association between subjective symptoms and psychological status. This relationship may be bidirectional, as symptoms may worsen anxiety and depression, while anxiety and depression may induce more severe symptoms (35). The results of this study showed that chest pain was a salient factor for anxiety and depression. Patients with chest pain tend to experience negative emotions and a decreased quality of life irrespective of the cause of the pain since they commonly attribute the discomfort to the nodules and associate it with adverse outcomes (6,31,36). These findings call for more attention to chest pain, especially noncardiac chest pain among patients with PNs. However, noncardiac chest pain is a subjective symptom with various etiologies, with the most prevalent being gastroesophageal reflux disease, which is easy to diagnose and has effective treatment options (37). In our study, we discovered that 13% of patients with chest pain had a history of pneumonia, but the role of pleurisy needs further confirmation. As mentioned above, psychogenic chest pain should not be neglected since psychological disorders also account for the development of chest pain and many other symptoms.
In this study, there was no significant difference in anxiety or depression related to gender or smoking status, which is contradictory to the results of previous studies. Kosson et al. (8) and Xiao et al. (4) reported that the prevalence of anxiety and depression was higher in women than in men. In our study, although PN detection had a psychological impact on both male and female patients, we did not explore the extent of the impact on the psychological burden according to gender, which may explain why we could not determine why female patients were more likely to be anxious and depressed. Moreover, in our study only 7.5% of the patients were current smokers, and thus the statistical power to assess the role of smoking status was very limited. Interestingly, the number of PNs was associated with psychological burden, while the size of the PNs was not. It is possible that the proportion of patients with nodules ≤10 mm in our study was too high to mask the level of anxiety and depression in patients with nodules >10 mm. This discrepancy could also be partially due to the patients’ lack of knowledge about PNs, as patients may not have a precise idea of the size of the nodule (6).
Our study also examined the impact of psychological status and other factors on PN management. We postulated that surveillance of potentially malignant PNs within a defined time frame is safe and appropriate based on clinical guidelines, and the preference of surgical or other medical treatment instead of follow-up only were defined as aggressive treatment strategies. In our study, just about half of patients preferred the “watch and wait” strategy, while those with abnormal anxiety or depression scores were less likely to choose this conservative surveillance plan. Given that most nodules grow indolently, longitudinal surveillance is widely recommended to avoid overtreatment of benign or premalignant diseases (2,21,22). On the other hand, guidelines also emphasize the importance of eliciting patient preferences (16), which is a prerequisite for good doctor-patient communication. However, patients’ treatment preferences are influenced by a variety of factors and are not fully understood. As mentioned above, patients with anxiety and depression tended to choose more radical and aggressive treatment modalities, which is consistent with the findings of other studies (15,25,38). In addition, our study also found that patients with subjective symptoms and progression of nodules tended to favor more aggressive strategies. In previous studies, doctor–patient communication and cancer risk were also significant influential factors on treatment preferences (39,40). It should be emphasized that an in-depth understanding of patients’ treatment preferences better assists in the management of PNs than does simply deferring to patients’ preferences. Deferring to the patient often leads to overtreatment, which results in misdiagnosis, removal of benign tumors, and a significant waste of medical resources. A high proportion of benign or precursor disease was reported in resected/biopsied lung tissue in patients who underwent aggressive management (12,13). This suggests that psychological status should be assessed in a timely manner and that other influential factors should be taken seriously to assist patients with making more informed decisions. Therefore, we propose that an ideal management strategy for PN management should balance guidelines and patient treatment preferences based on an objective analysis of nodules.
On a positive note, the adherence of patients in this study was remarkably high, although some patients thought the follow-up periods recommended by doctors were not appropriate. It is not surprising that patients with high levels of anxiety and depression were more willing to choose surgery. Clearly, doctors play a significant role in the medical behavior of patients. When doctors communicate with patients, they should not only explain the treatment and how it is administered, but also be attentive to the concerns and doubts of patients. Our results elucidate patients’ major concerns. For example, presence of cough was a risk factor for high levels of anxiety, and, similarly, patients with a family history of malignancy were more anxious. Implementing targeted patient education can promote healthy behaviors and alleviate negative emotions. As for patients with anxiety or depression, management strategies can be adjusted appropriately, such as by offering a shorter follow-up period. Surgery should also be included in the discussion when the patient is extremely distressed, especially for those nodules that can be wedge-resected. Of course, where necessary, psychosocial interventions should be implemented in collaboration with psychologists.
Limitations
Although this exploration has provided potentially valuable results, it has several limitations. First, our study was limited by its cross-sectional design. The lack of prospective studies raises concerns with regard to causal directionality, and thus findings need to be replicated within the context of longitudinal study designs. Second, anxiety and depression were measured using a self-reported scale without clinical diagnosis by psychiatrists. Although the HADS is convenient for research purposes, a questionnaire is not comparable with a formal psychiatric diagnosis of depression or anxiety. However, self-reports reflect patients’ subjective feelings and, nonetheless, assist with measuring patient concerns.
Conclusions
Anxiety and depression are common in patients with PNs, and they lead to a preference for more aggressive treatment. The factors influencing anxiety were age, subjective symptoms, family history of malignancy, number of PNs, and reason for CT workup, whereas depression was influenced by subjective symptoms, number of PNs, and the initial impression of doctors. Subjective symptoms significantly influenced treatment preferences. The results suggest that psychological status should be evaluated comprehensively to assist patients in making informed decisions.
Acknowledgments
Funding: This work was supported by the 2020 Guangdong Provincial Special Project for Popularization of Science and Technology Innovation (Grant No. 2020A1414070007); the Natural Science Foundation of Guangdong Province (Grant No. 2022A1515012469); and the Science and Technology Program of Guangzhou, China (Grant No. 202206010103).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-847/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-847/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-847/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-847/coif). GQ reports funding from the 2020 Guangdong Provincial Special Project for Popularization of Science and Technology Innovation (grant No. 2020A1414070007), the Natural Science Foundation of Guangdong Province (grant No. 2022A1515012469), and the Science and Technology Program of Guangzhou, China (Grant No. 202206010103). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. KY-Q-2021-005-03) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All the patients enrolled in this study provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Mazzone PJ, Lam L. Evaluating the Patient With a Pulmonary Nodule: A Review. JAMA 2022;327:264-73. [Crossref] [PubMed]
- Xiao R, Huang Y, Meng S, et al. A cross-sectional study of psychological burden in Chinese patients with pulmonary nodules: Prevalence and impact on the management of nodules. Thorac Cancer 2021;12:3150-6. [Crossref] [PubMed]
- Wu GX, Raz DJ, Brown L, et al. Psychological Burden Associated With Lung Cancer Screening: A Systematic Review. Clin Lung Cancer 2016;17:315-24. [Crossref] [PubMed]
- Freiman MR, Clark JA, Slatore CG, et al. Patients' Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J Thorac Oncol 2016;11:700-8. [Crossref] [PubMed]
- Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 2008;28:917-25. [Crossref] [PubMed]
- Kosson D, Malec-Milewska M, Gałązkowski R, et al. Analysis of Anxiety, Depression and Aggression in Patients Attending Pain Clinics. Int J Environ Res Public Health 2018;15:2898. [Crossref] [PubMed]
- Slatore CG, Golden SE, Ganzini L, et al. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules. A cohort study. Ann Am Thorac Soc 2015;12:184-92. [Crossref] [PubMed]
- Koroscil MT, Bowman MH, Morris MJ, et al. Effect of a pulmonary nodule fact sheet on patient anxiety and knowledge: a quality improvement initiative. BMJ Open Qual 2018;7:e000437. [Crossref] [PubMed]
- Li L, Zhao Y, Li H. Assessment of anxiety and depression in patients with incidental pulmonary nodules and analysis of its related impact factors. Thorac Cancer 2020;11:1433-42. [Crossref] [PubMed]
- Wiener RS, Gould MK, Slatore CG, et al. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med 2014;174:871-80. [Crossref] [PubMed]
- Tanner NT, Aggarwal J, Gould MK, et al. Management of Pulmonary Nodules by Community Pulmonologists: A Multicenter Observational Study. Chest 2015;148:1405-14. [Crossref] [PubMed]
- Zhuang W, Tang Y, Xu W, et al. Should psychological distress be listed as a surgical indication for indeterminate pulmonary nodules: protocol for a prospective cohort study in real-world settings. J Thorac Dis 2022;14:769-78. [Crossref] [PubMed]
- Wiener DC, Wiener RS. Patient-Centered, Guideline-Concordant Discussion and Management of Pulmonary Nodules. Chest 2020;158:416-22. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Wiener RS, Clark JA, Koppelman E, et al. Patient vs Clinician Perspectives on Communication About Results of Lung Cancer Screening: A Qualitative Study. Chest 2020;158:1240-9. [Crossref] [PubMed]
- Leung CM, Wing YK, Kwong PK, et al. Validation of the Chinese-Cantonese version of the hospital anxiety and depression scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand 1999;100:456-61. [Crossref] [PubMed]
- Leung CM, Ho S, Kan CS, et al. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J Psychosom 1993;40:29-34.
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17-41. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- He J, Li N, Chen WQ, et al. China guideline for the screening and early detection of lung cancer(2021, Beijing). Zhonghua Zhong Liu Za Zhi 2021;43:243-68. [Crossref] [PubMed]
- Andersson E, Dai Ydrefelt Y, Johannesson M, et al. Surveillance of indeterminate pulmonary nodules detected with CT in a Swedish population-based study (SCAPIS): psychosocial consequences and impact on health-related quality of life-a multicentre prospective cross-sectional study. BMJ Open 2021;11:e048721. [Crossref] [PubMed]
- Slatore CG, Wiener RS. Pulmonary Nodules: A Small Problem for Many, Severe Distress for Some, and How to Communicate About It. Chest 2018;153:1004-15. [Crossref] [PubMed]
- Hinz A, Finck C, Gómez Y, et al. Anxiety and depression in the general population in Colombia: reference values of the Hospital Anxiety and Depression Scale (HADS). Soc Psychiatry Psychiatr Epidemiol 2014;49:41-9. [Crossref] [PubMed]
- Han L. Prevalence, risk factors and prognostic role of anxiety and depression in surgical gastric cancer patients. Transl Cancer Res 2020;9:1371-83. [Crossref] [PubMed]
- Hamm AO. Fear, anxiety, and their disorders from the perspective of psychophysiology. Psychophysiology 2020;57:e13474. [Crossref] [PubMed]
- Rosenblat JD, Kurdyak P, Cosci F, et al. Depression in the medically ill. Aust N Z J Psychiatry 2020;54:346-66. [Crossref] [PubMed]
- Jacobson NC, Newman MG. Anxiety and depression as bidirectional risk factors for one another: A meta-analysis of longitudinal studies. Psychol Bull 2017;143:1155-200. [Crossref] [PubMed]
- Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med 2019;131:438-44. [Crossref] [PubMed]
- Nelson CJ, Weinberger MI, Balk E, et al. The chronology of distress, anxiety, and depression in older prostate cancer patients. Oncologist 2009;14:891-9. [Crossref] [PubMed]
- Mabuchi F, Yoshimura K, Kashiwagi K, et al. Risk factors for anxiety and depression in patients with glaucoma. Br J Ophthalmol 2012;96:821-5. [Crossref] [PubMed]
- Lee JM, Lowry KP, Cott Chubiz JE, et al. Breast cancer risk, worry, and anxiety: Effect on patient perceptions of false-positive screening results. Breast 2020;50:104-12. [Crossref] [PubMed]
- Leander M, Lampa E, Rask-Andersen A, et al. Impact of anxiety and depression on respiratory symptoms. Respir Med 2014;108:1594-600. [Crossref] [PubMed]
- Khairandish Z, Jamali L, Haghbin S. Role of anxiety and depression in adolescents with chest pain referred to a cardiology clinic. Cardiol Young 2017;27:125-30. [Crossref] [PubMed]
- Lenfant C. Chest pain of cardiac and noncardiac origin. Metabolism 2010;59:S41-6. [Crossref] [PubMed]
- Smith Z, Barnett SA, Gorelik A, et al. Strategies for the Management of Solitary Pulmonary Nodules: A Survey of Patient Preferences. Ann Thorac Surg 2022;113:1670-5. [Crossref] [PubMed]
- Moseson EM, Wiener RS, Golden SE, et al. Patient and Clinician Characteristics Associated with Adherence. A Cohort Study of Veterans with Incidental Pulmonary Nodules. Ann Am Thorac Soc 2016;13:651-9. [Crossref] [PubMed]
- Iaccarino JM, Simmons J, Gould MK, et al. Clinical Equipoise and Shared Decision-making in Pulmonary Nodule Management. A Survey of American Thoracic Society Clinicians. Ann Am Thorac Soc 2017;14:968-75. [Crossref] [PubMed]
(English Language Editors: B. Meiser and J. Gray)


