
Prognostic impact of pathologically confirmed rib invasion in patients with lung cancer requiring chest wall resection
Introduction
Chest wall invasion is found in 2% to 8% of patients with non-small cell lung cancer (NSCLC) undergoing surgical resection (1-3). Since the first report of en bloc excision of primary lung cancer invading the chest wall by Coleman (4) in 1947, multimodal treatments have been developed for locally advanced NSCLC (cT3N0-1 or T4N0-1) with chest wall invasion.
The eighth edition of the Tumour, Node, Metastasis (TNM) classification categorizes chest wall invasion as T3 disease regardless of the anatomical structures involved (parietal pleura only, intercostal soft tissue, or ribs). Although 5-year overall survival is reportedly worse in patients with pT3 NSCLC and chest wall invasion (25.0–67.0% in those with R0 resection and 8.0–21.0% in those with pN2 disease) (5,6), the literature contains little information on the prognostic impact of rib involvement in these patients. Moreover, because T3 disease requiring chest wall resection is not subdivided further, it is still not clear whether induction chemoradiotherapy or upfront surgery followed by adjuvant chemotherapy is best in these patients (7). Identification of the prognostic impact of chest wall invasion in patients with NSCLC and its optimal treatment is important for improvement of the outcomes in patients with pathological T3 disease requiring chest wall resection. We hypothesized that pathological rib invasion might be a poor prognostic factor among patients with T3 lung cancer requiring chest wall resection, including rib resection. The aim of this study was to investigate the prognostic impact of pathologically confirmed rib invasion in patients with NSCLC undergoing lung resection with en bloc resection of the chest wall. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-976/rc).
Methods
Patient selection
This retrospective cohort study was performed in the Department of Thoracic Surgery at Kyoto University Hospital. The electronic medical records of patients who underwent surgery for lung cancer from January 2006 to December 2019 were reviewed. Pathological data were registered according to the eighth edition of the TNM classification published by the Union for International Cancer Control.
The decision to administer induction chemoradiotherapy was made by members of a multidisciplinary tumor board after preoperative assessment of tumor resectability and the nodal status. After surgery, adjuvant chemotherapy was planned for the patients who had not received induction therapy. Pathological rib invasion was diagnosed in the cross section of the surgical specimen with the maximum chest wall invasion of lung cancer when cortical invasion of the rib by the tumor was identified in postoperative pathological examinations.
Follow-up computed tomography (CT) and laboratory investigations were scheduled every 6 months after surgery. When the CT findings suggested recurrence, fluorodeoxyglucose positron emission tomography was performed to guide treatment. Magnetic resonance imaging of the brain was performed when patients reported a symptom suspicious for brain metastasis.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Kyoto University Hospital (Approval Nos. E1668 and F2471), and the requirement for individual consent was waived because of the retrospective nature of the study.
Statistical analysis
Preoperative clinicopathological factors that potentially increased the risk of perioperative complications and the prognosis were examined in a logistic regression model. Categorical variables were compared between groups using the chi-squared test and continuous variables using the Mann-Whitney U test. Overall survival was calculated according to the Kaplan-Meier method and compared between groups using the log-rank test. All statistical analyses were performed using JMP Pro version 15.2.1 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant.
Results
Fifty-four patients underwent lung resection combined with chest wall resection during the study period. Ten patients with pT4 disease (pathological tumor size of >70 mm, vertebral invasion, and diaphragm invasion) and 2 patients with pT3N2 disease were excluded, leaving 42 patients with pT3-NSCLC who underwent chest wall resection for inclusion in the study (Figure 1). The median follow-up duration was 64.0 months.
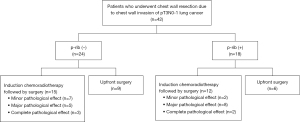
Patient characteristics, including sex, smoking status, and pulmonary function, are summarized in Table 1. The patients (41 male, 1 female) had a median age of 64 years (range, 42–79 years). The median tumor size before treatment was 56.5 mm (range, 21–80 mm). An osteolytic sign was identified on CT in 42.9% (18/42), and 27 (64.3%) of the 42 patients who underwent chest wall resection received induction chemoradiotherapy before surgery (Figure 2). The treatment regimen comprised cisplatin + vinorelbine or carboplatin + paclitaxel with radiotherapy (40–70 Gy). Eight patients clinically suspected to have N2 disease received induction chemoradiotherapy; preoperative positron emission tomography-CT showed disappearance of fluorodeoxyglucose uptake in the N2 lymph nodes in three patients, and a single N2 metastasis was suspected in the remaining five patients. Among eight patients suspected to have N2 disease, endobronchial ultrasound/transbronchial needle aspiration or mediastinoscopy was conducted to evaluate the N2 lymph nodes in five patients; all were proven negative. All surgeries were performed via open thoracotomy. The lung resections comprised 36 lobectomies, 5 segmentectomies, and 1 wedge resection. Chest wall resections were performed with resection of 2.5 ribs on average. Sublobar resection was selected in the patients without nodal metastasis to maximize operative tolerability based on assessment of the patients’ preoperative cardiopulmonary function. In these patients, lymph node dissection was performed to the same extent as in patients who underwent lobectomy. In three patients who required resection of more than three ribs, chest wall reconstruction was performed using a Marlex mesh or Gore-Tex patch closure. Squamous cell carcinoma was diagnosed in 22 (52.4%) patients, adenocarcinoma in 10 (23.8%), pleomorphic carcinoma in 5 (11.9%), and other types of cancer in 5 (11.9%).
Table 1
| Variables | Total, n=42 | Pathological rib invasion (+), n=18 |
Pathological rib invasion (−), n=24 |
P value |
|---|---|---|---|---|
| Age, years | 62.5±10.9 [42–79] | 64.6±10.2 [45–79] | 61.0±11.4 [42–77] | 0.2956 |
| Sex, M/F | 41/1 | 18/0 | 23/1 | 0.2862 |
| Smoking status, Y/N | 40/2 | 17/1 | 23/1 | 0.8351 |
| VC, mL | 3.75±0.82 [1.66–5.3] | 3.68±0.66 [2.5–5.3] | 3.80±0.93 [1.66–5.28] | 0.6236 |
| %VC | 97.8±19.9 [37.5–136.6] | 96.9±16.4 [67.3–129.5] | 98.5±22.5 [37.5–136.6] | 0.7976 |
| FEV1, mL | 2.72±0.70 [1.05–4.23] | 2.57±0.58 [1.05–3.45] | 2.84±0.77 [1.35–4.23] | 0.2278 |
| %FEV1 | 88.5±18.2 [40.4–121.0] | 83.6±16.5 [40.4–107.1] | 92.2±18.9 [60.8–121.0] | 0.1295 |
| Tumor size, mm | 55.6±14.5 [21–80] | 58.7±13.3 [31–80] | 53.3±15.2 [21–80] | 0.2429 |
| Clinical rib invasion | 17 | 11 | 6 | 0.0175 |
| Preoperative treatment | 27 | 10 | 17 | 0.3073 |
| Extent of lung resection, L/S/W | 36/5/1 | 13/5/0 | 23/0/1 | 0.0274, L vs. other |
| Histology, Sq/Ad/other | 22/10/10 | 12/2/4 | 10/8/6 | 0.1060, Sq vs. other |
| Pathological tumor size, mm | 33.7±22.9 [0–70] | 43.2±20.1 [10–70] | 26.6±22.6 [0–70] | 0.0184 |
| Pathological nodal status, N0/N1 | 38/4 | 15/3 | 23/1 | 0.1700, N0 vs. other |
| R0 | 39 | 16 | 23 | 0.3887 |
| Adjuvant therapy | 12 | 6 | 6 | 0.5552 |
Data are expressed as mean ± standard deviation [range] or number of patients. M, male; F, female; Y, yes; N, no; VC, vital capacity; FEV1, forced expiratory volume in 1 second; L, lobectomy; S, segmentectomy; W, wedge resection; Sq, squamous cell carcinoma; Ad, adenocarcinoma.
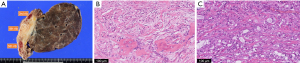
Pathological examinations revealed R0 resection in 39 (92.9%) of the 42 patients. Rib invasion was identified in 18 patients (pN0, n=15; pN1, n=3) but was absent in the remaining 24 patients (pN0, n=23; pN1, n=1) (Figure 3).
Analysis of postoperative complications associated with preoperative treatment
The postoperative complications were recorded using the Clavien-Dindo system. There was no mortality. Clavien-Dindo grade >IIIa postoperative complications occurred in 7 (16.7%) of 42 patients. Among 15 patients who underwent surgery without preoperative treatment, 1 patient developed chylothorax and another patient developed prolonged air leakage requiring pleurodesis. Three of 27 patients who underwent surgery following induction chemoradiotherapy developed persistent pleural effusion requiring repeat drainage, and 1 developed acute respiratory distress syndrome requiring intravenous steroids. The occurrence rate of Clavien-Dindo grade >IIIa postoperative complications was similar between patients with and without induction chemoradiotherapy (14.8% vs. 13.3%, respectively).
Comparison of recurrence patterns in patients with or without pathological rib invasion
The recurrence patterns are summarized in detail in Table 2. Local recurrence developed in the chest wall around the surgical margin, and growing abnormal shadows were confirmed on CT. Among six patients who underwent sublobar resection, recurrence was confirmed in five patients, and all recurrences were distant metastases. None of the four patients with N1 disease developed postoperative recurrence. The rates of locoregional recurrence and distant recurrence were significantly higher in patients with than without rib invasion (locoregional: 22.2% vs. 8.3%, distant: 33.3% vs. 12.5%, P=0.0073). None of the three patients with inadequate surgical margins who underwent postoperative radiotherapy to control local recurrence developed local relapse; however, one developed distant bone metastases. Among five patients who achieved a pathological complete response by induction therapy, two developed brain metastases.
Table 2
| Recurrence pattern | With pathological rib invasion (n=18) | Without pathological rib invasion (n=24) |
|---|---|---|
| Locoregional | 4 (22.2%): local (chest wall) 2; pleuritis 2 | 2 (8.3%): local (chest wall) 2 |
| Distant | 6 (33.3%): bone, n=3; upper arm, n=1; kidney, n=1; liver, n=1 | 3 (12.5%): brain, n=2; subauricular glands, n=1 |
Analysis of prognostic factors in patients with lung cancer requiring chest wall resection
The 5-year disease-free and overall survival rates of patients with and without clinically confirmed rib invasion on CT were 65.5% and 60.9% (P=0.8684) and 73.3% and 52.4% (P=0.2438), respectively (Figure 4). The 5-year disease-free and overall survival rates of patients with and without pathologically confirmed rib invasion were 44.4% and 77.4% (P=0.0114) and 44.7% and 81.3% (P=0.0222), respectively (Figure 5). A Cox proportional hazards model was used to identify potential prognostic factors. The factors used in this analysis were age, extent of lung resection, pathological tumor size, lymph node metastasis, and pathological rib invasion, all of which are generally recognized to be prognostic factors. The multivariate analysis identified postoperative rib invasion to be the only significant prognostic factor [hazard ratio (HR), 5.98; 95% confidence interval (CI): 1.37–26.1; P=0.0173] (Table 3).
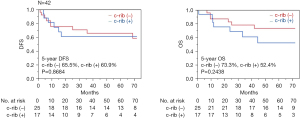
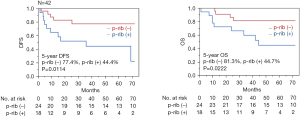
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | 1.02 | 0.97–1.08 | 0.4146 | 1.03 | 0.97–1.10 | 0.3592 | |
| Extent of lung resection | 1.40 | 0.31–6.34 | 0.6766 | 2.11 | 0.35–12.7 | 0.3989 | |
| Pathological size, mm | 1.00 | 0.98–1.03 | 0.6772 | 0.99 | 0.95–1.02 | 0.8110 | |
| Pathological rib invasion | 3.62 | 1.11–11.8 | 0.0244 | 5.98 | 1.37–26.1 | 0.0173 | |
| Pathological nodal status | 1.57 | 0.08–4.91 | 0.6440 | 3.62 | 0.40–32.5 | 0.2506 | |
HR, hazard ratio; CI, confidence interval.
Discussion
Although the eighth edition of the TNM classification categorizes NSCLC with chest wall invasion as pT3 regardless of the depth of invasion into anatomical structures of the chest wall (parietal pleura, intercostal muscles, and ribs), this study showed that pathological rib invasion was a significant unfavorable prognostic factor in patients with NSCLC requiring chest wall resection. Given that three patients who did not receive preoperative treatment were confirmed to have inadequate surgical margins and that both local and distant postoperative recurrence was more common in patients with pathological rib invasion, multimodal treatment (including preoperative chemoradiotherapy) may be required to achieve an adequate surgical margin and improve the prognosis in these patients. Regarding the potential future reclassification of T3 chest wall tumors, a recent retrospective study using a propensity score-matching analysis of 521 patients with pT3-T4 tumors by Zhao et al. (8) revealed that the 5-year overall survival rate of patients with pathological rib invasion was similar to that of patients with pT4 tumors, and the authors proposed rib invasion as an independent T description. Compared with a previous report from our institution (9), the 5-year overall survival rate of patients with pT4 tumors was 56.3%, and the prognosis of T3 disease with pathological rib invasion shown in the current study was similar to that of T4 disease.
The evidence to date indicates that completeness of resection including lobectomy and nodal involvement may be predictors of postoperative survival in patients with NSCLC who require chest wall resection. In one study, the 5-year survival rate was reported to be 32% in 175 patients with complete resection and 4% in 94 patients with incomplete resection (10). Furthermore, the survival rate ranges from 8.0% to 21.0% for patients with pN2 disease and from 25.0% to 67.0% for those with pN0 disease (11-14). Therefore, in addition to complete resection, preoperative mediastinal lymph node staging is recommended to exclude N2 disease, and if pathologically proven, the patient should receive multimodal therapy in view of the otherwise poor prognosis. Another study of patients with cT3N0 or cT3N1 NSCLC treated with induction chemoradiotherapy (two cycles of cisplatin at 80 mg/m2 + vinorelbine at 20 mg/m2 and 40-Gy irradiation) showed that 51% of patients achieved a partial response on CT after induction chemoradiotherapy and that 26% achieved a pathological complete response with a median 5-year survival rate of 62.6% (11). A further study of 2,326 patients who underwent upfront surgery that revealed pN0 (14) showed an association between adjuvant chemotherapy and longer survival (overall 5-year survival, 53% vs. 38%), which might suggest the importance of multimodal treatment for patients with chest wall invasion by NSCLC. Interestingly, in the current study, the locoregional recurrence rate after sublobar resection was relatively low. Even when lobectomy was deemed to be intolerable from the viewpoint of cardiopulmonary function, sublobar resection with chest wall resection would be potentially viable option for carefully selected patients (15).
Induction chemoradiotherapy has advantages when treating locally advanced NSCLC. First, it may achieve a higher rate of complete resection than surgery alone. Considering that en bloc resection is the primary goal for locally advanced NSCLC, ensuring adequate surgical margins can improve local control. Second, the response to chemotherapy, which is somehow modified by radiotherapy, can be assessed by pathological examination. These analyses facilitate the planning of postoperative treatment. However, the optimal timing of surgery may be missed if preoperative treatment does not achieve effective local control, and tumor progression or distant metastases may occur. Moreover, the tolerability of surgery following induction chemoradiotherapy may be decreased and the risk of postoperative complications increased because of impaired respiratory function (16). Although the morbidity rate associated with chest wall resection and reconstruction ranges from 24% to 46% and that for mortality ranges from 2% to 7% (11-14), there was no mortality in our study and Clavien-Dindo grade >IIIa postoperative complications occurred at a rate of 16.7%, which might be tolerable.
This study has several limitations that must be considered when interpreting its findings. First, the study had a retrospective cohort design and was based on data collected at a single center. Second, the number of patients was small. Third, the cohort was heterogeneous regarding perioperative treatment modalities. Because the chemotherapy regimen and radiation dose were not consistent, our finding that pathologically confirmed rib invasion has an unfavorable prognostic impact in patients with NSCLC needs confirmation in a future prospective study. A strict protocol including molecular targeted drug therapy and adjuvant immunotherapy will be required in future investigations of the prognostic impact of rib involvement in NSCLC that invades the chest wall.
Conclusions
This study showed that pathological rib invasion is a significant unfavorable prognostic factor in patients with NSCLC undergoing chest wall resection. Patients in whom rib invasion is clinically suspected before surgery may require multimodal treatment to improve their survival outcome.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-976/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-976/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-976/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-976/coif). MH serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Kyoto University Hospital (Approval Nos. E1668 and F2471), and the requirement for individual consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC, Greenberg JJ, Wilkins EW Jr. Resection of bronchogenic carcinoma involving thoracic wall. J Thorac Cardiovasc Surg 1966;51:417-21.
- Voltolini L, Rapicetta C, Luzzi L, et al. Lung cancer with chest wall involvement: predictive factors of long-term survival after surgical resection. Lung Cancer 2006;52:359-64. [Crossref] [PubMed]
- Sakakura N, Mizuno T, Kuroda H, et al. The eighth TNM classification system for lung cancer: A consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer 2018;118:134-8. [Crossref] [PubMed]
- Coleman FP. Primary Carcinoma of the Lung, with Invasion of the Ribs: Pneumonectomy and Simultaneous Block Resection of the Chest Wall. Ann Surg 1947;126:156-68. [Crossref] [PubMed]
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [Crossref] [PubMed]
- Magdeleinat P, Alifano M, Benbrahem C, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg 2001;71:1094-9. [Crossref] [PubMed]
- Facciolo F, Cardillo G, Lopergolo M, et al. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001;121:649-56. [Crossref] [PubMed]
- Zhao M, Wu J, Deng J, et al. Proposal for Rib invasion as an independent T descriptor for non-small cell lung cancer: A propensity-score matching analysis. Lung Cancer 2021;159:27-33. [Crossref] [PubMed]
- Yamanashi K, Menju T, Hamaji M, et al. Prognostic factors related to postoperative survival in the newly classified clinical T4 lung cancer. Eur J Cardiothorac Surg 2020;57:754-61. [Crossref] [PubMed]
- Downey RJ, Martini N, Rusch VW, et al. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999;68:188-93. [Crossref] [PubMed]
- Kawaguchi K, Yokoi K, Niwa H, et al. A prospective, multi-institutional phase II study of induction chemoradiotherapy followed by surgery in patients with non-small cell lung cancer involving the chest wall (CJLSG0801). Lung Cancer 2017;104:79-84. [Crossref] [PubMed]
- Ahmad U, Crabtree TD, Patel AP, et al. Adjuvant Chemotherapy Is Associated With Improved Survival in Locally Invasive Node Negative Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:303-7. [Crossref] [PubMed]
- Loi M, Mazzella A, Desideri I, et al. Chest wall resection and reconstruction for lung cancer: surgical techniques and example of integrated multimodality approach. J Thorac Dis 2020;12:22-30. [Crossref] [PubMed]
- Drake JA, Sullivan JL, Weksler B. Adjuvant chemotherapy improves survival in patients with completely resected T3N0 non-small cell lung cancer invading the chest wall. J Thorac Cardiovasc Surg 2018;155:1794-802. [Crossref] [PubMed]
- Yutaka Y, Sonobe M, Kawaguchi A, et al. Prognostic impact of preoperative comorbidities in geriatric patients with early-stage lung cancer: Significance of sublobar resection as a compromise procedure. Lung Cancer 2018;125:192-7. [Crossref] [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Is pulmonary function damaged by neoadjuvant lung cancer therapy? A comprehensive serial time-trend analysis of pulmonary function after induction radiochemotherapy plus surgery. J Thorac Cardiovasc Surg 2010;139:1457-63. [Crossref] [PubMed]


