
Long-term outcomes and risk factors for mortality of patients with hypertrophic obstructive cardiomyopathy undergoing septal myectomy
Introduction
Septal myectomy (SM) is one of the gold standard treatments for symptomatic hypertrophic obstructive cardiomyopathy (HOCM) refractory to medical treatment with low operative risk (<1%) (1-4). Several studies recently found that preoperative atrial fibrillation (AF) has an influence on the outcomes for patients with HOCM (5). In a large study of 3,673 patients with HOCM, preoperative AF was an independent risk factor for overall mortality (6). Another study of 1,557 consecutive patients with HOCM found that AF was not a major cause of death in HOCM, including sudden death, or of heart failure morbidity; however, it was associated with increased risk of embolic events (7). Determining whether or not AF has an impact on survival is important to surgeons because it may guide the decision to perform a concurrent surgical ablation. We aimed to evaluate the long-term clinical and echocardiographic outcomes of the standardized surgical treatment of HOCM from a single-center experience and to elucidate the risk factors for mortality, especially those related to AF. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1045/rc).
Methods
Study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-1060). Informed consent from the patients was not required owing to the retrospective nature of the study. We retrospectively reviewed 150 consecutive patients with HOCM (age 53±14 years, 48.7% male) who underwent surgical treatment at our institution between March 2003 and December 2020. The diagnosis of hypertrophic cardiomyopathy (HCM) was confirmed by echocardiographic imaging of septal hypertrophy, defined as a maximal septal thickness of at least 13 mm. Those who had a resting pressure gradient >30 mmHg on preoperative transthoracic echocardiography (TTE) or a provoked pressure gradient of 50 mmHg in the TTE were classified as having left ventricular outflow tract obstruction (LVOTO). Patients who received heart transplantation, concomitant aortic valve replacement (AVR), or simple mitral valve replacement (MVR) were excluded. A retrospective review of medical records was performed to identify patients eligible for inclusion in the study.
Surgical procedures
Median sternotomy with total cardiopulmonary bypass (CPB) using single or bicaval venous and aortic cannulation was used and the aorta was cross-clamped. SM was performed through transaortic, transapical, or transmitral access or a combination of these approaches. In the transaortic approach, antegrade cold blood cardioplegia was induced, and aortotomy was performed. Subsequently the extent of septal hypertrophy was visually estimated after retraction of the aortic valve leaflets. In the transapical approach, cold blood cardioplegia was induced via root cannula inserted into the proximal ascending aorta. The apex of the heart was oriented anteriorly and ventriculotomy was performed over the apical dimple 1 to 2 cm lateral and parallel to the left anterior descending coronary artery to avoid coronary compromise while performing closure during ventriculotomy. In the transmitral approach, cold blood cardioplegia was induced via a root cannula inserted into the proximal aorta, and left atriotomy was performed. Subsequently, the anterior leaflet of the mitral valve was incised and detached at its base to provide wide exposure of the ventricular septum. SM procedures were performed through either traditional Morrow SM or extended SM. For the former procedure, a standard rectangular myectomy was performed from 1 cm below the aortic valve apically to a point beyond the line of the mitral-septal contact and intraventricular obstruction, allowing relief of the outflow tract gradient and preservation of the atrioventricular node and left bundle branch. For the latter procedure, an extended incision was made apically to the bases of the papillary muscles and leftward back toward the mitral valve. Thereafter, the apical third of the right side of the septum was resected to obtain a much wider trough at the apex than at the base. After SM, additional mitral valve intervention or replacement was performed as needed. Relief of the left ventricular outflow tract (LVOT) pressure gradient was evaluated via intraoperative transesophageal echocardiography (TEE) or determination of left ventricular (LV) and aortic pressure; patients were then weaned off CPB, and wound closure was performed. Concomitant modified maze procedures were performed in 29 patients (19.3%). The details of modified maze procedures have been described in a previous study (8).
Outcomes and variables
The primary outcome was all-cause mortality, which was classified as early mortality (within 30 days of surgery) or late mortality (at least 30 days after surgery). Survival analysis was based on the results of a direct follow-up survey or indirectly through the current status of the mandatory national insurance coverage, which is deactivated only upon death or emigration. In addition, baseline characteristics, echocardiographic parameters, and operative profiles were obtained from the institutional electronic database. Echocardiographic parameters were collected at the following points: preoperative, immediate postoperative, and latest follow-up. To evaluate differences in surgical procedures, patients were categorized into three groups: isolated SM, SM with mitral valve intervention (SM + MVI), and SM with MVR (SM + MVR). The SM + MVI group was defined as having undergone concomitant procedures at the mitral valve or subvalvular apparatus other than MVR along with SM. To evaluate the impact of surgical ablation for AF, we collected information related to the maze procedure, and all data from 12-lead electrocardiogram (ECG) or 24-hour ambulatory ECG during the follow-up period.
Statistical analysis
Continuous variables are presented as means ± standard deviations or, for variables with a non-normal distribution, as median and interquartile ranges. The Student’s t-test or the Mann-Whitney U-test were used to compare continuous variables in two groups, and the Kruskal-Wallis test was used when comparing all three groups. Categorical variables are presented as percentages and frequencies. The χ2 or the Fisher’s exact test were used to compare categorical variables. Changes in echocardiographic parameters over time were analyzed using a generalized estimating equation. The 1-, 5-, and 10-year survival rates were estimated by Kaplan-Meier analysis. Log-rank tests were used to compare survival curves among the different groups. Univariable and multivariable Cox regression analyses were used to investigate the predictors of late mortality. A P value of ≤0.1 in the univariable analysis was used to select variables for multivariable analysis. A P value <0.05 was considered to indicate statistical significance. The critical level of significance was adjusted with the Bonferroni correction in case of multiple testing and subgroup analysis. Analyses were performed using IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
A total of 150 patients with HOCM were included in the study. Baseline characteristics are summarized in Table S1. The mean age was 53±14 years, and 73 (48.7%) patients were male. There were 30 (20.0%) patients who had persistent AF. The most common type of HOCM was basal (73.3%), followed by diffuse (22.7%).
Procedure information
All patients underwent SM. Fifty (33.3%, age 53.7±16.1 years) patients underwent isolated SM, 79 (52.7%, age 52.3±12.6 years) underwent SM + MVI, and 21 (14.0%, age 57.1±13.5 years) underwent SM + MVR. The most common MVI procedures were papillary muscle release (55/150, 36.7%), followed by anterior mitral valve leaflet plication (37/150, 24.7%), and mitral ring annuloplasty (6/150, 4.0%) (Table 1). The mean CPB and mean aortic cross clamping (ACC) times in the study were 133.2±67.1 and 78.6±44.6 min, respectively. Forty-two patients underwent CPB ≥2 times, and the resected myocardial mass through SM was 8.7±6.1 g (Table 1). CPB and ACC times in patient who underwent MVI (135±68, 72±40 min) or MVR (154±75, 97±52 min) were longer in patients who underwent isolated SM (122±61, 72±40 min); however, these differences were not statistically significant (P=0.2 and P=0.16, respectively). A similar trend was observed in the proportion of patients who underwent CPB ≥2 times (20.0%, isolated SM; 29.1%, SM + MVI; 42.9%, SM + MVR; P=0.14).
Table 1
| Operative details | Values |
|---|---|
| Approach | |
| Transaortic | 143 (95.3) |
| Transmitral | 26 (17.3) |
| Transapical | 13 (8.7) |
| Combined | 32 (21.3) |
| Transaortic + transmitral | 21 (14.0) |
| Transaortic + transapical | 11 (7.3) |
| Concomitant procedures | |
| Mitral valve replacement | 21 (14.0) |
| Mitral valve intervention | 79 (52.7) |
| Papillary muscle release | 55 (36.7) |
| Papillary muscle re-approximation | 1 (0.7) |
| Anterior mitral valve leaflet plication | 37 (24.7) |
| Chordae cutting | 4 (2.7) |
| Mitral ring annuloplasty | 6 (4.0) |
| Neochordae formation | 5 (3.3) |
| Alfieri | 5 (3.3) |
| Triangular resection | 5 (3.3) |
| Pericardial patch augmentation | 3 (2.0) |
| Cleft repair | 2 (1.3) |
| Other valvular surgery* | 7 (4.7) |
| Coronary artery bypass graft | 3 (2.0) |
| Maze operation | 29 (19.3) |
| ACC time, min | 78.6±44.6 |
| CPB time, min | 133.2±67.1 |
| Resected myocardial mass, g | 8.8±6.1 |
Values are n (%) or mean ± SD. *, including aortic valvuloplasty, tricuspid valvuloplasty, and tricuspid valve replacement. ACC, aortic cross clamping; CPB, cardiopulmonary bypass; SD, standard deviation.
Echocardiographic parameters during the study period
Echocardiographic parameters at preoperative, postoperative [median postoperative day (POD) 4], and latest follow-up (median POD 626 days) are shown in Table 2. Following surgical correction, all echocardiographic parameters except early to late diastolic transmitral flow velocity ratio (E/E’) ratio were significantly improved. Comparing between preoperative and latest echocardiographic data, ejection fraction and left atrium (LA) size were decreased [66.0%±4.9% to 63.3%±5.8% (P<0.001), 47.1±7.9 to 44.6±7.9 mm (P<0.001), respectively]. The peak LVOT pressure gradient at rest was significantly decreased from 91.9±43.2 to 13.3±13.0 mmHg (P<0.0001).
Table 2
| Echocardiographic data | Preoperative | Postoperative | Latest follow-up | P value |
|---|---|---|---|---|
| Ejection fraction, % | 66.0±4.9 | 62.4±5.8 | 63.3±5.8 | <0.001 |
| Left ventricular internal dimension systolic, mm | 23.9±4.3 | 28.7±5.8 | 28.7±6.4 | <0.001 |
| Left ventricular internal dimension diastolic, mm | 42.7±5.2 | 43.1±5.7 | 44.4±5.8 | 0.002 |
| Left atrium diameter, mm | 47.1±7.9 | 44.3±6.5 | 44.6±7.9 | <0.001 |
| Posterior wall of left ventricle systolic, mm | 18.0±3.2 | 17.4±3.2 | 16.3±2.9 | <0.001 |
| Posterior wall of left ventricle diastolic, mm | 12.0±2.7 | 11.8±2.5 | 10.9±2.4 | <0.001 |
| Interventricular septum systolic, mm | 22.2±4.7 | 17.4±4.4 | 17.3±4.5 | <0.001 |
| Interventricular septum diastolic, mm | 18.4±4.9 | 14.2±4.2 | 14.1±4.1 | <0.001 |
| Mitral regurgitation | <0.001 | |||
| Grade 0 | 11 (7.3) | 55 (37.2) | 54 (40.6) | |
| Grade 1 | 40 (26.7) | 70 (47.3) | 50 (37.6) | |
| Grade 2 | 37 (24.7) | 21 (14.2) | 24 (18.0) | |
| Grade 3 | 48 (32.0) | 1 (0.7) | 4 (3.0) | |
| Grade 4 | 14 (9.3) | 1 (0.7) | 1 (0.8) | |
| Left ventricular outflow tract pressure gradient at rest, mmHg | 91.9±43.2 | 19.7±15.3 | 13.3±13.0 | <0.001 |
Values are n (%) or mean ± SD.
Postoperative outcomes
Early outcomes
There were five early deaths. Three patients died from multiple organ failure due to bleeding, one from acute respiratory failure, and one from cerebral hemorrhage. Left bundle branch block developed in 84 patients and complete atrioventricular block developed in three patients, resulting in the need for permanent pacemaker insertion. Implantable cardioverter defibrillator therapy was performed in four patients for ventricular tachycardia or ventricular fibrillation. Five patients required repeat surgery within a postoperative period of 30 days due to bleeding, LV free wall rupture, and residual mitral regurgitation (MR) (Table 3).
Table 3
| Complications | Values |
|---|---|
| Early outcomes | |
| Operative mortality | 5 (3.3) |
| Early complications | |
| Complete atrioventricular block | 3 (2.0) |
| Left bundle branch block | 82 (54.7) |
| Ventricular septal defect | 2 (1.3) |
| Permanent pacemaker | 3 (2.0) |
| Ventricular tachycardia or fibrillation | 4 (2.7) |
| Bleeding control | 2 (1.3) |
| Left ventricular free wall rupture | 1 (0.7) |
| Severe mitral regurgitation | 2 (1.3) |
| Late outcomes | |
| Late mortality | 15 (10.0) |
| Late complications | |
| Severe mitral regurgitation | 2 (1.3) |
Values are n (%).
Late outcomes
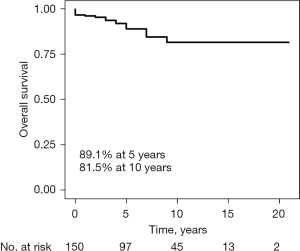
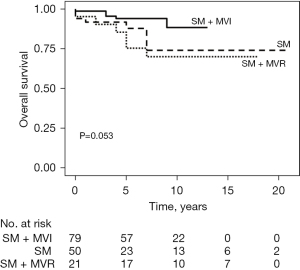
During the follow-up period, averaging 6.9±4.9 years, there were 15 late deaths and two patients needed repeat surgery due to severe MR (Table 3). One patient developed paravalvular leakage and one aggravated MR due to chordae rupture. The overall survival rates were 96.7%, 89.1%, and 81.5% at 30 days, 5 years, and 10 years, respectively (Figure 1). Patients in the SM + MVI group showed a higher overall survival rate than those in the SM + MVR or isolated SM groups with marginal statistical significance (94.1% vs. 75.4% vs. 88.0% at 5 years, respectively, P=0.05) (Figure 2).
Association between preoperative AF and late mortality
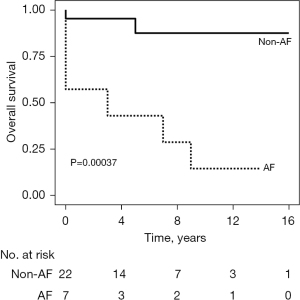
Thirty (20.0%) patients had persistent AF at baseline. Compared with patients without preoperative AF, patients with AF were older (52±14 vs. 58±12 years, P=0.04) (Table S2), had a larger LA diameter (45.5±6.5 vs. 53.2±9.9 mm, P<0.001) and a thicker LV posterior wall (17.7±3.0 vs. 19.2±3.6 for systolic, P=0.04; 11.7±2.4 vs. 13.1±3.4 for diastolic, P=0.03) (Table S3). Patients with preoperative AF had a worse 5-year survival rate than those without AF (73.4% vs. 92.8%, P<0.001) (Figure 3). Multivariable analysis showed that preoperative AF was an independent risk factor for late mortality, after adjusting for confounding factors including age, history of hypertension, LV internal dimensions, and surgical procedures [hazard ratio (HR), 3.26; 95% confidence interval (CI): 1.04–10.22, P=0.04] (Table 4).

Table 4
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | 1.06 (1.01–1.11) | 0.01 | 1.04 (0.98–1.10) | 0.16 | |
| Male | 1.91 (0.65–5.59) | 0.24 | – | – | |
| History of hypertension | 2.44 (0.87–6.87) | 0.09 | 1.71 (0.44–6.58) | 0.38 | |
| History of diabetes | 2.33 (0.53–10.34) | 0.27 | – | – | |
| Preoperative AF | 2.70 (0.92–7.90) | 0.06 | 3.26 (1.04–10.22) | 0.04 | |
| Left atrium (diameter) | 1.06 (0.99–1.13) | 0.12 | – | – | |
| LVID (systolic), mm | 1.13 (1.01–1.26) | 0.04 | 1.12 (1.00–1.26) | 0.05 | |
| IVST (systolic), mm | 0.94 (0.83–1.05) | 0.25 | – | – | |
| LVEF, % | 1.02 (0.92–1.12) | 0.73 | – | – | |
| LVOT PG, mmHg | 1.00 (0.99–1.01) | 0.98 | – | – | |
| Surgical procedure | |||||
| SM + MVI | Reference | Reference | Reference | Reference | |
| SM | 2.19 (0.63–7.60) | 0.22 | 1.91 (0.53–6.79) | 0.32 | |
| SM + MVR | 3.41 (0.99–11.78) | 0.05 | 2.15 (0.61–7.61) | 0.24 | |
HR, hazard ratio; CI, confidence interval; AF, atrial fibrillation; LVID, left ventricular internal dimension; IVST, interventricular septum thickness; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; PG, pressure gradient; SM, septal myectomy; MVI; mitral valve intervention; MVR, mitral valve replacement.
Outcome of surgical ablation for AF
Among 30 patients with preoperative persistent AF, 29 patients underwent surgical ablation during SM. The CPB and ACC times were 165.6±60.0 and 92.6±35.7 min, respectively, which were significantly longer than 125.4±66.7 and 75.2±46.0 min for patients without concomitant surgical ablation (P=0.003 and P=0.03, respectively). During the postoperative rhythm monitoring of median 1,573 days (IQR, 733–2,947 days), 22 patients had AF converted to non-AF, including 21 patients to sinus rhythm and one patient to atrial triggered ventricular pacing rhythm. Seven patients had persistent AF. When all 12-lead or 24-hour ambulatory ECG data were reviewed, two patients had transitory changes in rhythm from AF to non-AF or vice versa, without change in AF status from the postoperative stage to the latest follow-up. Those who successfully converted from AF to non-AF rhythm with surgical ablation showed a higher 5-year survival rate of 87.7% than patients who failed to abort AF (28.6%) (Figure 4). Ablation procedures were performed only on the left side in 13 (44.8%) patients and on both sides in 16 (55.2%) patients. The left atrial auricle was preserved in 14 (48.3%) patients, resected in 12 (41.4%) patients, and internally obliterated in 2 (6.9%) patients; however, procedures were not statistically significantly different between patients with aborted AF and those with retained AF (Table S3).
Discussion
The current study including 150 adult patients with HOCM who underwent surgical treatment demonstrated favorable long term echocardiographic and clinical outcomes. Patients who underwent SM + MVI appeared to have better overall survival than those in the SM + MVR or isolated SM groups; however, the difference was not statistically significant (94.1% vs. 75.4% vs. 88.0%, at 5 years, respectively, P=0.05). Notably, patients with preoperative AF had a worse survival rate; however, those who successfully converted to non-AF after surgical ablation had improved overall survival rates compared to those without preoperative AF.
HCM occurs in 1 in 500 individuals in the general population (9). LVOTO occurs in approximately 25% of these patients, leading to clinical symptoms, deterioration of quality of life, and worsening prognosis (10). In our institution, we selected surgical procedures from the options of SM or SM + MVI or SM + MVR through both preoperative TTE, TEE, heart computed tomography (CT), and direct inspection during operation. Papillary muscle release was performed when there was extensive fusion of papillary muscles with the septum or LV free wall. Anterior mitral valve leaflet plication was performed when the anterior mitral valve leaflet exceeded 3 cm in length and was floppy and lax (11). Different varieties of concomitant interventions to the mitral valve were performed case by case considering morphology. Several recent studies including a ultrasound study of 2,107 patients with HOCM who had undergone MVR for LVOTO had worse outcomes than patients undergoing SM with MVR (12-14). The results of the present study are consistent with those of this previous study, although statistical significance was not achieved, most likely due to the small number of patients (Figure 2). The SM + MVI group appeared to show better outcomes compared to the isolated SM group (94.1% vs. 88.0% at 5 years and 88.4% vs. 74.1% at 10 years), although statistical significance was not observed (P=0.06). This could be linked to the significant proportion of severe MR in the isolated SM group (n=19, 38%). MR in HOCM mostly occurs as a result of systolic anterior motion (SAM), which is relieved through SM. However, MR can occur due to intrinsic mitral valve abnormalities with or without SAM (14). Concomitant MVI may improve the long-term outcome in those patients; however, further investigation with a large cohort is required.
AF is a common arrhythmia associated with HCM, reported in approximately 20% of patients, although its effect on survival is controversial (6,7). A recent large US study of 2,203 HOCM patients who underwent SM reported that 19.5% of patients with preoperative AF had increased mortality (adjusted HR, 1.36; 95% CI: 0.97–1.91; P=0.07) (5). Similarly, preoperative AF was found in 30 of 150 patients (20.0%) in the present study and its status as an independent risk factor for late mortality was consistent between the two studies (adjusted HR, 3.26; 95% CI: 1.04–10.22; P=0.04). It is plausible that AF induces diastolic dysfunction, left atrial enlargement, and intrinsic atrial fibrosis in patients with HOCM (15). Notably, the previous study found that patients with AF treated with surgical ablation had a better survival rate than patients with unablated AF in those followed for longer than 5 years (5). The current study was unable to evaluate treatment outcomes between patients who had AF surgically ablated and those who did not, as only one patient did not undergo surgical ablation. However, we found that the long-term outcomes of patients with AF and HOCM varied depending on postoperative ECG after surgical ablation. Patients who were successfully converted from AF to non-AF by surgical ablation had an improved survival rate of 87.7% at 5 years, whereas patients with persisting AF after surgical ablation had poor prognosis (less than 50% at 5 years; Figures 3,4). Therefore, active interventions are required to convert preoperative AF to non-AF, including concomitant maze operations in patients with HOCM who are scheduled for surgical treatment.
There are several limitations of this study. First, the study was conducted retrospectively in a single tertiary center. Second, the number of patients enrolled in this study was small. Above all, the number of patients with AF was insufficient to establish a conclusive result. Third, early mortality in our institution was 3.3%, which is higher than that reported from a US study of 1,337 patients with HOCM who underwent SM at the Mayo clinic (3). This could be attributed to a difference in surgical volume. According to a large nationwide study of patients with HOCM who underwent SM from the Nationwide Inpatient Sample registry, the overall postoperative mortality rate after SM was 5.9%, but less than 1% in high-volume, highly-experienced hospitals (16). Improvement in early mortality would be expected as the surgical volume of SM increases in our institution. Finally, our patients did not undergo gene analysis. Nevertheless, the current study shed light on the implications of surgical ablation for concurrent AF in patients with HOCM during myectomy by reviewing all longitudinal ECG data during the follow-up period.
In conclusion, surgical treatment of patients with HOCM showed favorable echocardiographic and clinical outcomes with long-term follow-up. Notably, preoperative AF is a significant risk factor for poor surgical outcome of patients with HOCM. Active surgical ablation to convert AF rhythm to non-AF in HOCM during myectomy can improve patient outcomes; however, larger studies are needed to verify our findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1045/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1045/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1045/coif). JBK serves as an unpaid editorial board member of Journal of Thoracic Disease from February 2021 to January 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-1060). Informed consent from the patients was not required owing to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liebregts M, Vriesendorp PA, Mahmoodi BK, et al. A Systematic Review and Meta-Analysis of Long-Term Outcomes After Septal Reduction Therapy in Patients With Hypertrophic Cardiomyopathy. JACC Heart Fail 2015;3:896-905. [Crossref] [PubMed]
- Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 2007;116:196-206; discussion 206. [Crossref] [PubMed]
- Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470-6. [Crossref] [PubMed]
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:3022-55. [Crossref] [PubMed]
- Cui H, Schaff HV, Dearani JA, et al. Does ablation of atrial fibrillation at the time of septal myectomy improve survival of patients with obstructive hypertrophic cardiomyopathy? J Thorac Cardiovasc Surg 2021;161:997-1006.e3. [Crossref] [PubMed]
- Siontis KC, Geske JB, Ong K, et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. [Crossref] [PubMed]
- Rowin EJ, Hausvater A, Link MS, et al. Clinical Profile and Consequences of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation 2017;136:2420-36. [Crossref] [PubMed]
- Lee JW, Choo SJ, Kim KI, et al. Atrial fibrillation surgery simplified with cryoablation to improve left atrial function. Ann Thorac Surg 2001;72:1479-83. [Crossref] [PubMed]
- Pantazis A, Vischer AS, Perez-Tome MC, et al. Diagnosis and management of hypertrophic cardiomyopathy. Echo Res Pract 2015;2:R45-53. [Crossref] [PubMed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295-303. [Crossref] [PubMed]
- Balaram SK, Ross RE, Sherrid MV, et al. Role of mitral valve plication in the surgical management of hypertrophic cardiomyopathy. Ann Thorac Surg 2012;94:1990-7; discussion 1997-8. [Crossref] [PubMed]
- Collis R, Watkinson O, O'Mahony C, et al. Long-term outcomes for different surgical strategies to treat left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Eur J Heart Fail 2018;20:398-405. [Crossref] [PubMed]
- Polanco AR, D'Angelo A, Shea N, et al. Impact of Septal Myectomy Volume on Mitral-Valve Replacement Rate in Hypertrophic Cardiomyopathy Patients. Cardiology 2020;145:161-7. [Crossref] [PubMed]
- Hong JH, Schaff HV, Nishimura RA, et al. Mitral Regurgitation in Patients With Hypertrophic Obstructive Cardiomyopathy: Implications for Concomitant Valve Procedures. J Am Coll Cardiol 2016;68:1497-504. [Crossref] [PubMed]
- Papavassiliu T, Germans T, Flüchter S, et al. CMR findings in patients with hypertrophic cardiomyopathy and atrial fibrillation. J Cardiovasc Magn Reson 2009;11:34. [Crossref] [PubMed]
- Panaich SS, Badheka AO, Chothani A, et al. Results of ventricular septal myectomy and hypertrophic cardiomyopathy (from Nationwide Inpatient Sample [1998-2010]). Am J Cardiol 2014;114:1390-5.


