Health-related quality of life in a multiracial Asian interstitial lung disease cohort
Introduction
Interstitial lung diseases (ILDs) are a heterogeneous group of pulmonary disorders characterized by varying patterns of lung parenchymal inflammation and fibrosis (1). Many ILDs, especially idiopathic pulmonary fibrosis (IPF), have a high symptom burden that has a major impact on health-related quality of life (HRQL) (2). HRQL questionnaires provide insights into the impact of disease burden and treatment effects, paving the way for more holistic and individualised care in these patients (3). This has led to greater application of such instruments in clinical practice and research in recent years (4).
A recent systematic review showed that the most commonly used HRQL questionnaires in IPF research worldwide were St George’s Respiratory Questionnaire (SGRQ), Short Form 36 (SF-36), King’s Brief ILD questionnaire (K-BILD) and EuroQoL (EQ5D) (4). However, only 20% of these research were conducted in Asian populations, and the HRQL used were mainly SGRQ and SF-36 (4). While these generic respiratory HRQLs have been validated in IPF and used as outcome measures in clinical trials (4), some of the symptoms assessed, such as wheezing, may not be applicable to a wide range of ILD patients, potentially resulting in a weaker association between the SGRQ symptoms domain and ILD severity (5).
The K-BILD was developed from a mixed group of ILD patients, including IPF, connective tissue disease-related ILD (CTD-ILD), hypersensitivity pneumonitis (HP) and cystic lung diseases, and has been shown to have high concurrent validity with SGRQ and SF-36 (6). To date, no studies have described the HRQL of ILD patients using K-BILD in an Asian population (4). A recently published research statement recommended using a combination of disease-specific and generic HRQL questionnaires in ILD research (7). In Asian populations, the EQ5D 3-level (EQ5D-3L) is the preferred choice of preference-based measure (8), and it is commonly used in health economic evaluations to estimate quality-adjusted life-years (QALYs) to determine the cost-effectiveness of interventions (9). With this background, we aim to describe the HRQL of a multiracial, Asian ILD patient cohort using K-BILD and EQ5D-3L. A secondary aim is to investigate the associations between the K-BILD and EQ5D-3L scores and various clinical variables. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-906/rc).
Methods
Study design and population
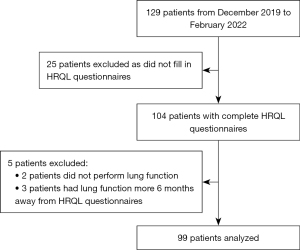
Data were collected from a cross-sectional study of patients who attended the ILD clinic in a university-affiliated tertiary public hospital in Singapore between December 2019 and February 2022. Patients who were over 21 years old and diagnosed with ILD according to prevailing international guidelines (1) were invited to participate in the study. Demographics and lung function indices were retrieved from electronic medical records, and patients were invited to complete 2 self-administered HRQL questionnaires upon study entry. ILD disease severity was assessed using the GAP index, which is a staging system based on total scores obtained from the patient’s gender, age, forced vital capacity (FVC) and carbon monoxide diffusing capacity (DLCO) percentage predicted (%pred) values (10). GAP Stage I corresponds to the mildest disease whereas Stage III is the most severe ILD associated with the worst prognosis. Patients must have lung function tests performed within 6 months of the HRQL questionnaires to be included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was granted by the National Healthcare Group Domain Specific Review Board (Reference No. 2019/00894) as part of a larger study on examining the long-term outcomes of ILD patients. All patients provided written informed consent before study entry.
HRQL questionnaires
A generic HRQL questionnaire, EQ5D-3L, and an ILD-specific HRQL questionnaire, K-BILD, were administered. The English and Mandarin versions of both questionnaires were used.
EQ5D-3L
The 3-level version of the EQ5D comprises 2 components: the EQ5D descriptive system and the visual analogue scale (VAS). The EQ5D-3L descriptive system assesses 5 dimensions: ‘mobility’, ‘self-care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’. For each dimension, patients rate their health state on a 3-point Likert scale. A 5-digit number (from 11111 to 33333) is derived by combining the scores from the dimensions (11). This is translated into a utility value based on the EQ5D-3L value set for Singapore that represents the health state of the patient. The score ranges from −0.769 to 1.000, with negative values being worse than death, 0 representing death and 1.000 representing the best health state (12). The VAS allows patients to rate their health on a vertical visual analogue scale with endpoints ‘best imaginable health state’ (100) at the top of the scale and ‘worst imaginable health state’ (0) at the base of the scale (11).
K-BILD
The K-BILD is a 15-item ILD-specific questionnaire that evaluates 3 domains: ‘breathlessness and activities’, ‘psychological’ and ‘chest symptoms’. For each item, the patient rates their health state on a 7-point Likert scale (6). Based on a predetermined scoring algorithm, responses are weighted and combined to produce a total score and 3 domain scores. The scores are converted to a range of 0 to 100 based on a logit transformation, with higher scores representing a better HRQL (13).
Assessment of co-variables
Clinical factors deemed to potentially influence HRQL were assessed based on previously published data. This includes baseline demographics, ILD diagnosis, comorbidities, measures of ILD severity such as the modified Medical Research Council dyspnoea scale (mMRC) and lung function indices including FVC and DLCO %pred values (14-16). The Charlson comorbidity index (CCI) was used as a measure of comorbidity burden, as it has been shown to affect outcomes in ILD patients in various studies (17,18). It consists of a summed score of 19 comorbid conditions, including cardiac diseases, peripheral vascular disease, cerebrovascular accident, dementia, chronic obstructive lung disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, chronic kidney disease, haematological and solid organ malignancy and acquired immunodeficiency syndrome, each weighted according to its potential influence on mortality (19).
Data collection and statistical analysis
Descriptive statistics were generated for baseline demographics, comorbidities, disease severity and HRQL measures. Between-group comparisons were performed with Fisher’s exact test, the Chi-squared test, Student’s t-test or the Mann-Whitney U test where appropriate. To assess for any potential selection bias, we compared the baseline characteristics of patients who completed the HRQL questionnaires to those who did not. Spearman’s correlational analysis was conducted between the EQ5D-3L utility value, VAS, the domain and total scores for K-BILD, as well as the CCI, mMRC scores and FVC and DLCO %pred values. We considered correlations of <0.3 as weak, 0.3 to 0.59 as moderate, 0.6 to 0.79 as strong and ≥0.8 as very strong (20). Separate classical or robust multiple linear regression analyses with robust standard errors were performed to investigate the effects of influencing factors on the HRQL questionnaire results. The independent variables examined were age, sex, race, smoking history, body mass index, ILD diagnosis, FVC %pred, DLCO %pred and CCI. For VAS, K-BILD total, ‘breathlessness and activities’ and ‘psychological’ domains scores, classical ordinary Least Square estimation was used in the multiple regression analysis. Due to violation of assumption of homoscedasticity and presence of extreme but genuine values observed in the data points for EQ5D-3L utility value and K-BILD ‘chest symptoms’ domain, robust regression technique using MM-estimator was used to estimate the multiple linear regression coefficients of the model for each of these two dependent variables. In the presence of outliers, the use of robust regression techniques tends to improve efficiency and reduce bias in comparison to using classical ordinary least square estimation (21). Missing FVC and DLCO %pred data were imputed on the basis that mMRC scores were significantly associated with FVC and DLCO values and reflect the severity of the underlying ILD (22). For FVC %pred, data were imputed based on the mean FVC %pred for the mMRC category the subject is in. For subjects with missing DLCO %pred with missing absolute FVC values or FVC >1 litre, DLCO %pred was imputed based on the mean DLCO %pred for the mMRC category the subject is in. If the subject had an FVC <1 litre, the DLCO %pred was imputed using the lowest DLCO value as patients were not able to perform the DLCO measurements and hence will have low DLCO values. There were no missing data for the other variables. Data were analysed with SPSS Version 28.0.0.0 (IBM Corp. Armonk, NY, USA) and Stata 14.2 (StataCorp LP. College Station, TX, USA). Robust MM estimation was performed using the robreg module in Stata (23). A P value of ≤0.05 was considered statistically significant.
Results
Patient characteristics
Out of the 129 patients from December 2019 to February 2022, 104 patients (81%) completed both HRQL questionnaires. Five patients (2 with no lung function tests and 3 had lung function tests performed more than 6 months from the HRQL questionnaires) were excluded. Ninety-nine patients were included in the final analysis (Figure 1). Table 1 shows the baseline characteristics of the study population. The median (interquartile range (IQR)) age was 63 (54–72) years. Patients were predominantly male (n=55, 56%), Chinese (n=74, 75%) and never smokers (n=63, 64%). The most common ILD diagnosis was CTD-ILD (n=51, 52%), followed by IPF (n=27, 27%). Out of the patients with CTD-ILDs, the three most common CTDs were idiopathic inflammatory myopathies (n=21, 41%), followed by systemic sclerosis (n=11, 22%) and overlap syndromes (n=6, 12%). In terms of diagnosis, 62 patients (63%), including all of the IPF patients, were diagnosed via the ILD multi-disciplinary meeting. The rest of the ILDs, with the majority being CTD-ILDs (31/37, 84%), were physician diagnosed. Eleven patients (11%) required a surgical lung biopsy to aid in ILD diagnosis, and only 1 IPF patient (4%) underwent a surgical lung biopsy. There were no patients with acute ILDs such as acute interstitial pneumonia. In the whole cohort, 32 patients (32%) had the usual interstitial pneumonia (UIP) pattern on the high resolution computed tomography scan of the chest. In the CTD-ILD group, only 3 patients (6%) had a UIP pattern. Thirteen patients (13%) had no pre-existing comorbidities, while 10 patients (10%) had ≥5 comorbidities. In terms of healthcare utilization, the bulk of the patients had not previously been hospitalised (n=61, 62%), and 12 patients (12%) had ≥3 hospitalizations. Seven patients (7%) received long-term oxygen therapy, and 14 patients (14%) underwent pulmonary rehabilitation. Fourteen patients (14%) were on anti-fibrotic therapy, and 53 patients (54%) were on immunosuppressive therapy. There was no difference in terms of baseline demographics, comorbidities, ILD diagnosis or disease severity between those patients who answered the HRQL questionnaires and those who did not (Table S1).
Table 1
| Characteristics | Total sample (N=99) |
|---|---|
| Male sex | 55 [56] |
| Age, years | 63 [54–72] |
| Race | |
| Chinese | 74 [75] |
| Malay | 11 [11] |
| Indian | 8 [8] |
| Others | 6 [6] |
| Body mass index, kg/m2 | 24.3 (4.3) |
| Smoking history | |
| Never smoker | 63 [64] |
| Ex-smoker | 27 [27] |
| Current smoker | 9 [9] |
| ILD diagnosis | |
| CTD-ILD | 51 [52] |
| Idiopathic inflammatory myopathies | 21 [41] |
| Systemic sclerosis | 11 [22] |
| Overlap syndromes | 6 [12] |
| Rheumatoid arthritis | 4 [8] |
| Others† | 9 [18] |
| IPF | 27 [27] |
| CHP | 5 [5] |
| Cystic lung diseases | 5 [5] |
| Unclassifiable | 4 [4] |
| Others‡ | 7 [7] |
| Long term oxygen therapy | 7 [7] |
| Number of comorbidities | |
| None | 13 [13] |
| 1 | 20 [20] |
| 2 | 34 [34] |
| 3 | 10 [10] |
| 4 | 12 [12] |
| 5 or more | 10 [10] |
| Number of hospitalisations | |
| None | 61 [62] |
| 1 | 17 [17] |
| 2 | 9 [9] |
| 3 or more | 12 [12] |
| Pulmonary rehabilitation | 14 [14] |
| ILD specific treatments | |
| Anti-fibrotic therapy | 14 [14] |
| Immunosuppressive therapy | 53 [54] |
Categorical data is presented as count [percentage] and continuous data is presented as mean (standard deviation) or median [interquartile range] depending on distribution. †, Includes 4 patients with primary Sjogren’s syndrome, 3 patients with systemic lupus erythematosus and 2 patients with mixed connective tissue disease. ‡, Includes 1 patient each with diagnosis of drug-induced ILD, smoking related ILD, familial ILD, pulmonary alveolar proteinosis, sarcoidosis, pleuroparenchymal fibroelastosis and Erdheim-Chester disease. ILD, interstitial lung disease; CTD-ILD, connective tissue disease-related interstitial lung disease; IPF, idiopathic pulmonary fibrosis; CHP, chronic hypersensitivity pneumonitis.
ILD severity, comorbidities, lung function and HRQL measures
We analysed the symptoms, comorbidities, lung function and HRQL measures in the various ILD subgroups: CTD-ILD, IPF and other ILDs (Table 2). Using IPF as the reference group, there was no significant difference in the median (IQR) mMRC scores between the various ILD subgroups [1 (0–2) in IPF, 1 (0–2) in CTD-ILD and 0 (0–3) in other ILDs]. The majority of the patients were GAP stage I (n=63, 64%), and 7 (7%) patients belonged to stage III. The CTD-ILD group had a milder severity (82% GAP stage I, 12% GAP stage II, 2% GAP stage III) than the IPF patients (33% GAP stage I, 52% GAP stage II, 15% GAP stage III). The median (IQR) CCI was 3 (2–4), with no sub-group differences. The mean [standard deviation (SD)] FVC was 2.31 (0.73) L and 79.8 (21.6) %pred, and DLCO was 4.40 (1.33) mmol/kPA.min and 59.8 (19.9) %pred. The CTD-ILD group had a higher DLCO than the IPF group [63.8 (20.4) vs. 50.7 (14.4) %pred, P=0.004]. The median (IQR) duration between the lung function tests and the completion of HRQL questionnaires was 0.5 (0–123) days.
Table 2
| Characteristics | Total population | IPF | CTD-ILD | Other ILDs |
|---|---|---|---|---|
| Sample size | 99 | 27 [27] | 51 [52] | 21 [21] |
| mMRC score† | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0 (0–3) |
| GAP index‡ | ||||
| Stage I | 63 [64] | 9 [33] | 42 [82]* | 12 [57] |
| Stage II | 24 [24] | 14 [52] | 6 [12]* | 4 [19] |
| Stage III | 7 [7] | 4 [15] | 1 [2]* | 2 [10] |
| CCI, median [interquartile range] | 3 [2–4] | 3 [3–5] | 3 [2–4] | 3 [1–4] |
| Lung function | ||||
| FVC§ (absolute, litres) | 2.31 (0.73) | 2.39 (0.73) | 2.20 (0.68) | 2.49 (0.84) |
| FVC§ (% predicted) | 79.8 (21.6) | 78.1 (20.5) | 80.2 (22.3) | 81.0 (22.4) |
| DLCO¶ (absolute, litres) | 4.40 (1.33) | 4.06 (1.09) | 4.47 (1.24) | 4.68 (1.74) |
| DLCO¶ (% predicted) | 59.8 (19.9) | 50.7 (14.4) | 63.8 (20.4)* | 61.7 (22.0) |
| HRQL EQ5D-3L | ||||
| Utility value | 0.806 (0.284) | 0.763 (0.425) | 0.828 (0.198) | 0.808 (0.242) |
| VAS | 75.1 (12.8) | 74.2 (11.6) | 76.9 (11.9) | 72.1 (15.7) |
| K-BILD | ||||
| Breathlessness & activities | 53.5 (20.3) | 50.5 (16.3) | 53.7 (20.8) | 56.9 (23.8) |
| Psychological | 66.8 (18.8) | 63.5 (18.7) | 68.2 (18.6) | 67.7 (19.8) |
| Chest symptoms | 76.6 (19.1) | 76.2 (21.6) | 77.4 (17.1) | 75.4 (21.0) |
| Total | 63.9 (14.3) | 61.7 (14.1) | 64.6 (14.3) | 64.9 (15.1) |
All values reported as mean (standard deviation) or number [frequency] unless otherwise stated. Only group comparison with P<0.05 are labelled (*) using IPF as a reference group. †, data reported as median (interquartile range). ‡, data missing for 2 patients for CTD-ILD and 3 patients for other ILDs; §, data missing for 1 patient for CTD-ILD and 2 patients for other ILDs; ¶, data missing for 2 patients for IPF, 3 patients for CTD-ILD and 2 patients for other ILDs; Unpaired T-test or Mann-Whitney U test with IPF as the reference group for comparison. IPF, idiopathic pulmonary fibrosis; CTD-ILD, connective tissue disease-related interstitial lung disease; ILD, interstitial lung disease; mMRC, modified medical research council dyspnoea scale; GAP, gender, age, physiology; CCI, Charlson’s comorbidity index; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; HRQL, health-related quality of life; EQ5D-3L, EuroQol 5-dimension 3 level version; VAS, visual analogue scale; K-BILD, King’s brief interstitial lung disease Questionnaire.
In terms of HRQL measures, the mean (SD) EQ5D-3L was 0.806 (0.284) for utility value and 75.1 (12.8) for VAS. The total K-BILD score was 63.9 (14.3), with the lowest scores in the ‘breathlessness and activities’ domain: 53.5 (20.3), followed by the ‘psychological’ domain: 66.8 (18.8) and the ‘chest symptoms’ domain: 76.6 (19.1). IPF patients had the lowest ED5D-3L utility values, K-BILD total and the ‘psychological’ and ‘breathlessness and activities’ domain scores compared with patients with CTD-ILD and other ILDs, but the difference was not statistically significant.
Participants with greater disease severity as stratified using the GAP stage had poorer HRQL scores on both the EQ5D-3L and K-BILD measurements (Table S2). In terms of the various ILD subtypes across the different GAP stages, there was no significant difference between the HRQL measures using IPF as the reference group (Table S2). GAP stages I and II CTD-ILD patients had numerically lower EQ5D-3L and K-BILD scores than IPF patients of the same GAP stages.
Correlation between HRQL measures and co-variables
Table 3 shows the correlation between the two HRQLs, disease severity measures and CCI. Overall, EQ5D-3L showed a moderate correlation with the K-BILD total and domain scores, with r=0.538 (P<0.001) between the EQ5D-3L utility value and the K-BILD total. Between the K-BILD domains and EQ5D-3L utility value, the ‘breathlessness and activities’ domain had the strongest correlation (r=0.547, P<0.001). The EQ5D VAS also correlated moderately with the K-BILD domains, with the strongest correlation being the ‘breathlessness and activities’ domain (r=0.470, P<0.001).
Table 3
| Variables | EQ5D-3L utility value | EQ5D-3L VAS | K-BILD total | K-BILD breath | K-BILD psych | K-BILD chest | mMRC score | FVC† | DLCO† | CCI |
|---|---|---|---|---|---|---|---|---|---|---|
| EQ5D-3L utility value | 1 | |||||||||
| EQ5D-3L VAS | 0.530**& | 1 | ||||||||
| K-BILD total | 0.538**& | 0.471**& | 1 | |||||||
| K-BILD breath | 0.547**& | 0.470**& | 0.950**&& | 1 | ||||||
| K-BILD psych | 0.470**& | 0.420**& | 0.952**&& | 0.830**&& | 1 | |||||
| K-BILD chest | 0.489**& | 0.382**& | 0.840**&& | 0.772**&& | 0.756**&& | 1 | ||||
| mMRC score | −0.535**& | −0.525**& | −0.585**& | −0.581**& | −0.535**& | −0.475**& | 1 | |||
| FVC† | 0.336**& | 0.266** | 0.396**& | 0.389**& | 0.375**& | 0.213* | −0.527**& | 1 | ||
| DLCO† | 0.218* | 0.275** | 0.414**& | 0.448**& | 0.360**& | 0.260** | −0.437**& | 0.569**& | 1 | |
| CCI | −0.187 | −0.220* | −0.185 | −0.228* | −0.162 | −0.128 | 0.314**& | −0.013 | −0.185 | 1 |
Correlation 0.8= very strong, 0.6–0.79= strong, 0.3–0.59= moderate, <0.3= weak. &&, highlight very strong or strong correlation; &, moderate correlation. *, correlation is significant at the 0.05 level (2-tailed); **, correlation is significant at the 0.01 level (2-tailed); †, percentage predicted values. EQ5D-3L, EuroQol 5-dimension 3 level version; K-BILD, King’s brief interstitial lung disease questionnaire; K-BILD breath, K-BILD breathlessness & activities; K-BILD psych, K-BILD psychological; K-BILD chest, K-BILD chest symptoms; mMRC, modified medical research council dyspnoea scale; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; CCI, Charlson comorbidity index; VAS, visual analogue scale.
The mMRC score was moderately correlated with the K-BILD total (r=−0.585, P<0.01), the K-BILD domain scores (r=−0.475 to −0.581, P<0.01) and the EQ5D-3L utility values (r=−0.535, P<0.01) and VAS (r=−0.525, P<0.01). The %pred FVC and DLCO showed a weak-to-moderate correlation with most of the HRQL instrument measures. The FVC %pred correlated most strongly with the K-BILD total score (r=0.396, P<0.001) and moderately correlated with the EQ5D-3L utility value (r=0.336, P=0.001). The correlation of %pred DLCO with HRQL measures was at best moderate, with the highest correlation with the K-BILD ‘breathlessness and activities’ domain (r=0.448, P<0.001), but it was weakly correlated with the VAS (r=0.275, P=0.007) and EQ5D-3L utility values (r=0.218, P=0.015). In terms of comorbidities, there was a weak correlation between CCI and the VAS (r=−0.220, P=0.029) and K-BILD ‘breathlessness and activities’ (r=−0.228, P=0.023) domains and a moderate correlation with mMRC scores (r=0.314, P<0.001).
Association between HRQL measures and clinical variables
In the multiple linear regression analyses (Table 4), DLCO %pred was an independent predictor of low HRQL measures of the K-BILD total, the K-BILD ‘breathlessness and activities’ domain and K-BILD ‘psychological’ domain when adjusted for age, sex, race, smoking history, body mass index, ILD diagnosis, CCI and FVC %pred. Non-Chinese race was a significant predictor of higher scores in the K-BILD ‘psychological’ domain compared with Chinese. None of the covariates was found to be predictive of low EQ-5D utility values, VAS scores or K-BILD ‘chest symptoms’ scores.
Table 4
| Covariate | HRQL measures | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EQ-5D-3L utility value† | EQ5D-3L VAS‡ | K-BILD total‡ | K-BILD breath‡ | K-BILD chest† | K-BILD psych‡ | |||||||||||||||||||||||||
| Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | |||||||||||||
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |||||||||||||||||||
| Age | 0.001 | −0.003 | 0.006 | 0.577 | −0.021 | −0.309 | 0.267 | 0.886 | −0.202 | −0.517 | 0.113 | 0.206 | −0.369 | −0.772 | 0.033 | 0.071 | −0.249 | −0.814 | 0.317 | 0.385 | −0.306 | −0.766 | 0.155 | 0.191 | ||||||
| Sex | ||||||||||||||||||||||||||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | 0.894 | |||||||||||||||||||||||
| Female | 0.064 | −0.066 | 0.184 | 0.1927 | 0.464 | −7.464 | 8.391 | 0.908 | 3.446 | −4.626 | 11.519 | 0.399 | 2.155 | −8.161 | 12.470 | 0.679 | 7.585 | −12.150 | 27.321 | 0.447 | 0.808 | −11.236 | 12.851 | |||||||
| Race | ||||||||||||||||||||||||||||||
| Chinese | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||||||||||||
| Non-Chinese | 0.031 | −0.044 | 0.107 | 0.414 | 3.630 | −2.401 | 9.660 | 0.235 | 6.008 | −0.388 | 12.404 | 0.065 | 8.987 | −0.276 | 18.250 | 0.057 | 7.634 | −2.327 | 17.595 | 0.131 | 8.680 | 0.656 | 16.704 | 0.034 | ||||||
| Smoking history | ||||||||||||||||||||||||||||||
| Never Smoker | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||||||||||||
| Ever Smoker | 0.028 | −0.061 | 0.118 | 0.529 | 0.242 | −6.591 | 7.075 | 0.944 | 4.172 | −3.383 | 11.726 | 0.275 | 5.896 | −4.094 | 15.886 | 0.244 | 3.302 | −10.811 | 17.416 | 0.643 | 2.722 | −6.727 | 12.171 | 0.568 | ||||||
| BMI | −0.004 | −0.013 | 0.004 | 0.315 | −0.192 | −0.795 | 0.411 | 0.528 | 0.023 | −0.544 | 0.590 | 0.937 | −0.330 | −1.029 | 0.370 | 0.351 | 0.065 | −1.057 | 1.187 | 0.909 | 0.089 | −0.732 | 0.911 | 0.829 | ||||||
| FVC %predicted | 0.000 | −0.002 | 0.003 | 0.450 | 0.092 | −0.073 | 0.258 | 0.270 | 0.093 | −0.069 | 0.256 | 0.258 | 0.063 | −0.170 | 0.296 | 0.591 | 0.075 | −0.287 | 0.438 | 0.682 | 0.162 | −0.053 | 0.377 | 0.137 | ||||||
| DLCO %predicted | 0.001 | −0.002 | 0.004 | 0.422 | 0.126 | −0.027 | 0.280 | 0.105 | 0.244 | 0.075 | 0.414 | 0.005 | 0.448 | 0.192 | 0.703 | 0.001 | 0.217 | −0.129 | 0.562 | 0.216 | 0.256 | 0.024 | 0.488 | 0.031 | ||||||
| ILD diagnosis | ||||||||||||||||||||||||||||||
| Other ILD | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||||||||||||
| CTD-ILD | −0.015 | −0.124 | 0.095 | 0.792 | 4.955 | −2.367 | 12.278 | 0.182 | −2.494 | −10.427 | 5.438 | 0.534 | −5.464 | −15.224 | 4.295 | 0.269 | −3.492 | −17.396 | 10.411 | 0.619 | −1.742 | −13.835 | 10.350 | 0.775 | ||||||
| IPF | 0.088 | −0.011 | 0.186 | 0.082 | 5.435 | −2.379 | 13.250 | 0.170 | 0.893 | −7.209 | 8.994 | 0.827 | 1.486 | −9.720 | 12.691 | 0.793 | 6.153 | −11.149 | 23.455 | 0.482 | 0.367 | −9.857 | 10.590 | 0.943 | ||||||
| Charlson Comorbidity Index | −0.010 | −0.046 | 0.027 | 0.601 | −0.835 | −2.292 | 0.623 | 0.258 | 1.166 | −0.693 | 3.026 | 0.216 | 1.108 | −1.201 | 3.418 | 0.343 | 1.421 | −2.072 | 4.913 | 0.421 | 1.614 | −0.922 | 4.149 | 0.209 | ||||||
†, Robust regression: MM estimator (75% efficiency) with robust standard errors; ‡, Classical ordinary Least Square linear regression with robust standard errors. HRQL, health-related quality of life; EQ5D-3L, EuroQol 5-dimension 3 level version; K-BILD, King’s brief interstitial lung disease questionnaire; K-BILD breath, K-BILD breathlessness & activities; VAS, visual analogue scale; K-BILD chest, K-BILD chest symptoms; K-BILD psych, K-BILD psychological; CI, confidence interval; LB, lower bound; Ref, reference; UB, upper bound; BMI, body mass index; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; ILD, interstitial lung disease; CTD, connective tissue disease; IPF, idiopathic pulmonary fibrosis.
Discussion
In this study, we described the HRQL in a diverse sample of ILD patients in a multiracial Asian population using the ILD-specific K-BILD questionnaire and the generic EQ5D-3L. We found that HRQL in these patients is significantly impaired, and unsurprisingly, IPF has the lowest quality of life when compared to other ILD diagnosis. However, when stratified by disease severity, the low HRQL in IPF appears to be driven by GAP stage III patients, whereas CTD-ILD patients had poorer HRQL in GAP stages I and II. We also demonstrated a moderate correlation between the K-BILD total and domain scores with the EQ5D-3L utility value and VAS. Similarly, these HRQL measures have a moderate correlation with ILD severity measures such as mMRC scores and lung function. Lower DLCO %pred is an independent predictor of poorer HRQL as measured by the K-BILD questionnaire.
Overall, our study population has better HRQL scores than existing local or overseas cohorts (14,24). The most likely explanation is the milder disease severity in our patient population, as evidenced by the higher FVC and DLCO %pred values compared with other studies, with almost two-thirds of our patients being GAP Stage I (25). Consistent with other studies, IPF patients have the poorest HRQL scores in terms of EQ5D-3L utility value, K-BILD total, ‘psychological’, ‘breathlessness and activities domain’ compared to CTD-ILD and other ILD patients (26,27). This could partly be explained by the lower DLCO values in IPF patients, which was a significant determinant of poor HRQL in our study. Moreover, IPF has the poorest survival (median survival of 2.5 to 3.5 years) (28) compared with CTD-ILDs (29) and other ILDs (30) with a highly variable clinical disease course (28). Uncertainty in prognosis can have a significant impact on a patient’s mental health, which in turn would negatively affect the patient’s HRQL (31). Intriguingly, when the HRQL of the various ILD subtypes is stratified according to disease severity, GAP stage I and II CTD-ILD patients, even though not statistically significant, had lower HRQL scores compared with IPF patients. This could be because of the extrapulmonary manifestations of CTD that have been found to be significantly correlated with lower HRQL measurements (32).
The use of the ILD-specific K-BILD questionnaire and the generic EQ5D-3L questionnaires complement each other in our study. This approach of combining disease-specific and generic HRQL questionnaires is recommended by the recent American Thoracic Society research statement on patient-centred outcome research in ILD (7). K-BILD assesses symptoms such as breathlessness, chest tightness and also the impact of these symptoms on the individual. It has the advantage of being brief (15 items) compared to the other disease-specific HRQL questionnaires such as SGRQ (50 items) or A Tool to Assess Quality of Life in IPF (ATAQ-IPF), which had 72 items (7). EQ5D-3L is a generic HRQL questionnaire that has been used in ILD studies, and health-adjusted life-years can be measured from these results (4). In addition, EQ5D-3L allows comparisons for HRQL across patients with different disease conditions. This has value both in the research setting, and in policy planning such as health economic evaluation studies and burden of disease estimates. EQ5D-3L assesses the overall health status of the patient, such as mobility, self-care and daily activities, rather than respiratory disease-specific symptoms. Hence, the EQ5D-3L utility value and health status score reflect the combined effects of the underlying respiratory disease, extrapulmonary manifestations (in the case of CTDs) and comorbidities (4,7). It is perhaps for this reason that DLCO %pred was not a predictor of EQ5D-3L scores as it was just one of the many aspects that contributed to the HRQL of these patients.
The mMRC scores showed a moderate correlation with both HRQL instruments. Previously published studies have found the severity of dyspnoea to be a major determinant of HRQL in ILD patients (33,34). This is not surprising given that dyspnoea is the most common symptom of ILD and is linked to depression and poorer functional status (35). In contrast, the correlation between lung function and HRQL was weak to moderate at best. These findings are comparable to existing K-BILD validation studies, reaffirming that HRQL assesses aspects of disease impact that cannot be fully accounted for by isolated physiological parameters (14). Similar findings were demonstrated in studies using other HRQL tools (27,36-39). This approach allows clinical management to centre on the patients’ perspectives and provides information that cannot be substituted by clinical parameters alone.
We found that CCI has a weak correlation with HRQL measures. Contrary to other studies that examined comorbidities (14,15), it was not a significant predictor of poor HRQL in the multivariate regression analysis. This may be because CCI did not include comorbidities that are considered important in ILD, such as gastro-oesophageal reflux, pulmonary hypertension, arterial hypertension, pulmonary embolism, obstructive sleep apnoea and psychiatric conditions such as depression and anxiety (15,40). Indeed, one study revealed that arterial hypertension had a significant influence on a low EQ5D-3L VAS, and depression had a strong negative association with the K-BILD ‘chest symptoms’ domain (14). Moreover, the CCI was originally developed to predict mortality rather than to examine the impact of various comorbidities on the quality of life of a patient (19). Further studies are needed to standardize the list of comorbidities that will most affect the quality of life in ILD patients and to determine whether addressing these comorbidities will improve the HRQL in these patients.
Our study showed that the DLCO %pred, but not FVC %pred, is a significant predictor of poor K-BILD total, ‘psychological’ and ‘breathlessness and activities’ domains in ILD patients. Indeed, a lower baseline DLCO %pred is associated not only with poorer HRQL (25,34) but also with future HRQL deterioration (16,41). Even though the degree of reduction in DLCO usually mirrors the corresponding drop in FVC, a severe reduction in DLCO may represent the presence of pulmonary hypertension on top of lung parenchymal destruction from ILD, and pulmonary hypertension has been shown to decrease the quality of life in IPF patients (42). Likewise, co-existing emphysema with ILD will also cause a disproportionate reduction in DLCO %pred. Patients with combined pulmonary fibrosis and emphysema have a worse quality of life than those with IPF alone (43). Interestingly, non-Chinese race was an independent predictor of better K-BILD psychological domain scores. Singapore is a multi-racial society with majority consisting of Chinese, Malay and Indian populations, and ethnic specific impact on quality of life has been reported (44). Possible reasons include different variables affecting HRQL among the various racial groups and unmeasured determinants of HRQL due to differences in identity, culture, minority status, socioeconomic status and health perception (44).
Study limitations include selection bias as one-fifth of the patients did not complete the HRQL questionnaires. Some non-responders may lack literacy, which often results in the exclusion of certain patient groups, such as those with existing disabilities and lower socioeconomic status. However, the missing data analysis did not find any significant differences in the baseline demographics and ILD severity among the responders and non-responders to the questionnaires, and the frequency of patients with a diagnosed psychiatric disorder was very low (4% in the responder group and none in the non-responder group). The relatively small sample size may also explain the lack of independent predictors associated with lower EQ-5D-3L utility values, VAS or K-BILD “chest symptoms” scores. In addition, we did not evaluate other factors that can contribute negatively to HRQL in ILD, such as socioeconomic status, employment status, education level, symptoms such as cough, fatigue, and measurements of functional status, such as the 6-minute walk test (7). Finally, due to the cross-sectional study design, we were unable to examine the influence of disease-modifying treatments, support from allied health (e.g., ILD nurse) or palliative care interventions that may affect the patient’s HRQL over time (31).
Conclusions
The use of the K-BILD and EQ5D-3L questionnaires in ILD patients provides patient-centred information that is not captured by routine physiological measures. Clinicians should consider using it in routine clinical settings to provide more holistic care to patients. Particular attention should be given to ILD patients with low DLCO, as they may have poor HRQL. Early referral to supportive management, such as pulmonary rehabilitation or palliative care, may improve symptoms, HRQL, physical functioning and emotional well-being in these patients (31). Cross-cultural validation and translation of the K-BILD questionnaires to local languages such as Malay and Tamil are needed to reach the other native speakers to examine whether race truly affects the HRQL in ILD patients. Further work using qualitative methodologies to explore the determinants of higher K-BILD psychological domain scores in non-Chinese racial groups is recommended.
Acknowledgments
We would like to thank Ms Sheryl Ng Hui Xian from the National Healthcare Group Health Services and Outcomes Research Department for her suggestions and edits to the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-906/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-906/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-906/coif). GTC reports honoraria for lectures and advisory board fees from Boehringer Ingelheim (Singapore) Pte Ltd. paid to his institution. GTC is the Honorary Secretary in Chapter of Respiratory Medicine, Academy of Medicine, Singapore. Geak Poh Tan received Tan Tock Seng Hospital 2019 Pitch-for-fund grant for unrelated project. GPT holds the position of vice chairman of the Chapter of Respiratory Medicine, Academy of Medicine, Singapore. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was granted by the National Healthcare Group Domain Specific Review Board (Reference No. 2019/00894) as part of a larger study on examining the long-term outcomes of ILD patients. All patients provided written informed consent before study entry.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Moor CC, Heukels P, Kool M, et al. Integrating Patient Perspectives into Personalized Medicine in Idiopathic Pulmonary Fibrosis. Front Med (Lausanne) 2017;4:226. [Crossref] [PubMed]
- Kalluri M, Luppi F, Vancheri A, et al. Patient-reported outcomes and patient-reported outcome measures in interstitial lung disease: where to go from here? Eur Respir Rev 2021;30:210026. [Crossref] [PubMed]
- Cox IA, Borchers Arriagada N, de Graaff B, et al. Health-related quality of life of patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur Respir Rev 2020;29:200154. [Crossref] [PubMed]
- Swigris JJ, Esser D, Conoscenti CS, et al. The psychometric properties of the St George's Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes 2014;12:124. [Crossref] [PubMed]
- Patel AS, Siegert RJ, Brignall K, et al. The development and validation of the King's Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax 2012;67:804-10. [Crossref] [PubMed]
- Aronson KI, Danoff SK, Russell AM, et al. Patient-centered Outcomes Research in Interstitial Lung Disease: An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2021;204:e3-e23. [Crossref] [PubMed]
- Qian X, Tan RL, Chuang LH, et al. Measurement Properties of Commonly Used Generic Preference-Based Measures in East and South-East Asia: A Systematic Review. Pharmacoeconomics 2020;38:159-70. [Crossref] [PubMed]
- Abdin E, Subramaniam M, Vaingankar JA, et al. Population norms for the EQ-5D index scores using Singapore preference weights. Qual Life Res 2015;24:1545-53. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Luo N, Wang P, Thumboo J, et al. Valuation of EQ-5D-3L health states in Singapore: modeling of time trade-off values for 80 empirically observed health states. Pharmacoeconomics 2014;32:495-507. [Crossref] [PubMed]
- Patel AS, Siegert RJ, Bajwah S, et al. Rasch analysis and impact factor methods both yield valid and comparable measures of health status in interstitial lung disease. J Clin Epidemiol 2015;68:1019-27. [Crossref] [PubMed]
- Szentes BL, Kreuter M, Bahmer T, et al. Quality of life assessment in interstitial lung diseases:a comparison of the disease-specific K-BILD with the generic EQ-5D-5L. Respir Res 2018;19:101. [Crossref] [PubMed]
- Kreuter M, Swigris J, Pittrow D, et al. Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Respir Res 2017;18:139. [Crossref] [PubMed]
- Kreuter M, Wuyts WA, Wijsenbeek M, et al. Health-related quality of life and symptoms in patients with IPF treated with nintedanib: analyses of patient-reported outcomes from the INPULSIS® trials. Respir Res 2020;21:36. [Crossref] [PubMed]
- Yagyu H, Murohashi K, Hara Y, et al. Clinical utility of a composite scoring system including Charlson Comorbidity Index score in patients with interstitial lung disease. J Thorac Dis 2020;12:5774-82. [Crossref] [PubMed]
- Murohashi K, Hara Y, Saigusa Y, et al. Clinical significance of Charlson comorbidity index as a prognostic parameter for patients with acute or subacute idiopathic interstitial pneumonias and acute exacerbation of collagen vascular diseases-related interstitial pneumonia. J Thorac Dis 2019;11:2448-57. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Akoglu H. User’s guide to correlation coefficients. Turkish J Emerg Med. 2018;18:91-3. [Crossref] [PubMed]
- Alma ÖG. Comparison of Robust Regression Methods in Linear Regression. Int J Contemp Math Sci 2011;6:409-21.
- Papiris SA, Daniil ZD, Malagari K, et al. The Medical Research Council dyspnea scale in the estimation of disease severity in idiopathic pulmonary fibrosis. Respir Med 2005;99:755-61. [Crossref] [PubMed]
- Ben Jann 2021. ROBREG: Stata module providing robust regression estimators. Statistical Software Components S458930, Boston College Department of Economics, revised 19 Feb 2022.
- Tan YH, Nor MM, Kam MLW, et al. Impact of pulmonary rehabilitation in patients with interstitial lung disease in Singapore. Ann Acad Med Singap 2021;50:349-52. [Crossref] [PubMed]
- O'Brien EC, Hellkamp AS, Neely ML, et al. Disease Severity and Quality of Life in Patients With Idiopathic Pulmonary Fibrosis: A Cross-Sectional Analysis of the IPF-PRO Registry. Chest 2020;157:1188-98. [Crossref] [PubMed]
- Durheim MT, Hoffmann-Vold AM, Eagan TM, et al. ILD-specific health-related quality of life in systemic sclerosis-associated ILD compared with IPF. BMJ Open Respir Res 2020;7:e000598. [Crossref] [PubMed]
- Wapenaar M, Patel AS, Birring SS, et al. Translation and validation of the King's Brief Interstitial Lung Disease (K-BILD) questionnaire in French, Italian, Swedish, and Dutch. Chron Respir Dis 2017;14:140-50. [Crossref] [PubMed]
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705-11. [Crossref] [PubMed]
- Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J 2013;42:750-7. [Crossref] [PubMed]
- Antoniou K, Kamekis A, Symvoulakis EK, et al. Burden of idiopathic pulmonary fibrosis on patients' emotional well being and quality of life: a literature review. Curr Opin Pulm Med 2020;26:457-63. [Crossref] [PubMed]
- Allanore Y, Constans J, Godard D, et al. Quality of life in SSc-ILD patients: Understanding the impact of the ILD and the needs of the SSc-ILD patients and their need for caregivers in France. J Scleroderma Relat Disord 2022;7:49-56. [Crossref] [PubMed]
- Nagata K, Tomii K, Otsuka K, et al. Evaluation of the chronic obstructive pulmonary disease assessment test for measurement of health-related quality of life in patients with interstitial lung disease. Respirology 2012;17:506-12. [Crossref] [PubMed]
- Yuan XY, Zhang H, Huang LR, et al. Evaluation of health-related quality of life and the related factors in a group of Chinese patients with interstitial lung diseases. PLoS One 2020;15:e0236346. [Crossref] [PubMed]
- Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, et al. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest 2011;139:609-16. [Crossref] [PubMed]
- Prior TS, Hilberg O, Shaker SB, et al. Validation of the King's Brief Interstitial Lung Disease questionnaire in Idiopathic Pulmonary Fibrosis. BMC Pulm Med 2019;19:255. [Crossref] [PubMed]
- Swigris JJ, Kuschner WG, Jacobs SS, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax 2005;60:588-94. [Crossref] [PubMed]
- Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St George's Respiratory Questionnaire. Thorax 2010;65:921-6. [Crossref] [PubMed]
- Chang JA, Curtis JR, Patrick DL, et al. Assessment of health-related quality of life in patients with interstitial lung disease. Chest 1999;116:1175-82. [Crossref] [PubMed]
- Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev 2017;26:160027. [Crossref] [PubMed]
- Maqhuzu PN, Szentes BL, Kreuter M, et al. Determinants of health-related quality of life decline in interstitial lung disease. Health Qual Life Outcomes 2020;18:334. [Crossref] [PubMed]
- Yan W, Peng LY, Ban CJ, et al. Incidence and clinical characteristics of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chin Med J (Engl) 2015;128:896-901. [Crossref] [PubMed]
- Kim HJ, Snyder LD, Neely ML, et al. Clinical Outcomes of Patients with Combined Idiopathic Pulmonary Fibrosis and Emphysema in the IPF-PRO Registry. Lung 2022;200:21-9. [Crossref] [PubMed]
- Thumboo J, Fong KY, Machin D, et al. Quality of life in an urban Asian population: the impact of ethnicity and socio-economic status. Soc Sci Med 2003;56:1761-72. [Crossref] [PubMed]