Limiting the risk of cardiac toxicity with esophageal-sparing intensity modulated radiotherapy for locally advanced lung cancers
Introduction
For patients with inoperable, locally advanced non-small cell lung cancer (NSCLC) concurrent chemo-radiotherapy represents the standard of care (1). Although concurrent chemotherapy improves survival by approximately 5% at 5 years, it also significantly increases the risk of toxicity (2). Among the potential toxicities, pneumonitis and esophagitis are the best understood and for these reliable dose-volume constraints are routinely considered during radiotherapy planning (3,4).
Intensity modulated radiotherapy (IMRT) is routinely used for NSCLC today (5). The initial implementation of IMRT for NSCLC was to enable dose escalation without increasing the risk of pneumonitis and esophagitis. Grills et al. compared IMRT with optimized multi-angle 3D conformal radiotherapy (3DCRT), limited-angle 3DCRT and a traditional elective nodal irradiation technique (6). They found IMRT enabled dose escalation and reduced toxicity in patients with node positive cancer or tumors in close proximity to the esophagus. In node positive patients, IMRT was associated with reduced mean lung, mean esophagus and maximum esophagus dose, with corresponding reductions in normal tissue complication probabilities (NTCP). Al-Halabi investigated the ability of IMRT to further spare the esophagus by using a contralateral esophagus-sparing technique on 20 patients receiving a median dose of 70.2 Gy (7). Their technique resulted in no patients experiencing grade 3 or above toxicity.
Cardiac toxicity is far less considered during radiotherapy planning for locally advanced NSCLC and no clear organ at risk constraints exist (8). However, the recent findings of the RTOG 0617 study indicate the radiotherapy doses to the heart and resultant toxicity may be more significant than currently appreciated (5). Comparing 60 vs. 74 Gy with or without cetuximab, the authors found the volume of heart receiving ≥5 and ≥30 Gy to be independent predictors of survival. We performed a planning study to determine whether IMRT retained its ability to reduce the risk of pneumonitis and esophagitis when radiotherapy planning also considered reduction in cardiac doses.
Methods
3D conformal plans
This study was approved by The Alfred Ethics Committee (project no.88/15). Due to the retrospective nature of the work, we were granted a waiver of the requirement to provide information and obtaining patient consent in accordance with the National Health & Medical Research Council Act 1992. Between January 2012 to July 2014, ten consecutive patients with stage III N2 NSCLC were treated at Alfred Health Radiation Oncology with concurrent chemo-radiotherapy to 60 Gy in 30 fractions, delivered over six weeks. Patients were simulated supine with their arms above their head, supported by a personalized fixation device. In all cases the gross tumor volume (GTV) was delineated on CT and motion was considered using 4DCT or planning FDG-PET. A planning target volume (PTV) was generated by applying a 1–1.5 cm margin to consider microscopic disease and setup inaccuracies. Patients were treated using a conventional 3D conformal technique (3DCRT) with 6MV static fields typically made up of an anterior, posterior and one or two oblique fields. 3DCRT plans were prescribed to an ICRU reference point (9) and were optimized using wedges and beam weightings.
Intensity modulated plans
Inverse-planned IMRT plans were retrospectively generated utilizing static fields with a sliding window technique. The original prescription of 60 Gy in 30 fractions was maintained, but prescribed volumetrically as per ICRU recommendations, such that 100% of the prescribed dose covers 50% of the target volume (10). Beam number and arrangement were individualized to achieve adequate dose to the target volume, whilst minimizing healthy lung exposure. In general, beams entering through the contralateral lung and heart were avoided if possible and no beams opposed one another. All plans were optimized such that 95% of the PTV received ≥95% of the prescribed dose (D95% ≥57 Gy) and 99% of the PTV received ≥93% of the prescribed dose (D99% ≥55.8 Gy). IMRT plans were first created with the heart not outlined or considered during optimization (IMRT A). A second IMRT plan was then created, with the heart outlined and included in the optimization (IMRT B).
Planning for both 3DCRT and IMRT was carried out on the Eclipse Treatment Planning System v.11.0.31 (Varian Medical Systems, Palo Alto, CA). All dosimetric calculations were carried out using the Anisotropic Analytical Algorithm (AAA) with heterogeneity corrections.
Organs at risk considerations
Spinal cord
Dose to the spinal cord was kept below 45 Gy. To ensure that a high dose gradient did not occur too close to the organ, a planning risk volume (PRV) was generated incorporating the true spinal cord plus a margin of 4 mm to account for daily setup errors. This volume was used in the inverse optimizer with constraints limiting the dose received to below 45 Gy.
Healthy lung tissue
Healthy lung was delineated to include the paired lungs minus the GTV. In both planning techniques, this volume was used to evaluate the plan, whereby the volume receiving 5, 20 and 30 Gy was required to be below 60%, 30% and 20%, respectively (V20Gy <30%, V30Gy <20%, V5Gy <60%). In IMRT, this same volume was employed to limit the 20 and 30 Gy dose to the lung tissue during inverse optimization of the plan. In order to reduce the low dose received by the lungs and ensure compliance of the V5Gy constraint, a separate contour around the contralateral lung was generated and included in the inverse optimization. This approach limits the weighting of beams that exit out the contralateral lung, thereby placing more weighting on the remaining beams. For this reason, less conformality of the high dose to the PTV in the anteroposterior direction was accepted in favor of ensuring the V5Gy lung constraint was met.
Esophagus
The esophagus was contoured from the inferior edge of the cricoid cartilage down to the gastro-esophageal junction. Doses were optimized such that none of the esophagus received the prescription dose (i.e., 60 Gy) and volumes receiving 40–60 Gy were reduced to as low as possible. To achieve the former constraint, a contour encompassing the overlap region of the PTV with the esophagus plus a margin of 0.2 cm (extending only inside the PTV) was created. This contour enabled a region where the PTV minimum required dose could be maintained whilst restricting any dose higher than it, thereby protecting the esophagus from the high dose region. The 0.2 cm margin acts as a buffer for the esophagus and prevents the optimizer from placing a high dose gradient too close to the organ. In order to limit the amount of esophagus receiving >40 Gy, a structure was generated corresponding to the esophagus volume outside the PTV volume. This helped define a clear region for the optimizer to create a high dose gradient without compromising on PTV coverage.
Heart
The heart was contoured to the extent of the pericardial sac extending superiorly to the level where the right and left pulmonary arteries separate. Beam number and orientation were maintained between the two IMRT plans. The goal for these plans, in addition to the other organ at risk constraints, was to spare the heart from the prescription dose, while reducing the volume of heart receiving 5 and 30 Gy to be as low as possible. In order to spare the heart from the prescription dose a separate structure was created where the heart overlapped with the PTV plus a margin of 0.3 cm (extending only inside the PTV). This volume was used in the optimizer to restrict the amount of 60 Gy from being deposited. A similar technique was used for the low and intermediate doses, whereby the volume of heart minus PTV was used in optimization to reduce the V5Gy and V30Gy.
Statistical analysis
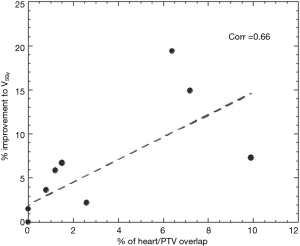
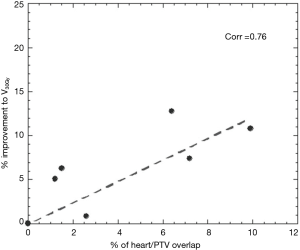
A paired t-test was used to compare the mean values of PTV D95%, PTV D99%, esophagus V60Gy, V50Gy and V40Gy, lung V5Gy, V20Gy and V30Gy and heart V5Gy and V30Gy between conventional 3DCRT and IMRT A and between IMRT A and IMRT B (α=0.05). Normal tissue control probability (NTCP) was calculated using the BioSuite software, designed for radiobiological analysis (11). A Lyman-Kutcher-Burman model was used to determine the probability of lung radiation pneumonitis (TD50 =29.2 Gy, m=0.45, n=1, α/β=3 Gy) (12) and acute esophageal toxicity grade ≥2 (TD50 =47 Gy, m=0.36, n=0.69, α/β=1.7 Gy) (13). A relative seriality model was instead used to calculate the probability of cardiac mortality (TD50 =70.3 Gy, g=0.96, s=1, α/β=3 Gy) (14). In the specific case of the heart a Pearson product-moment correlation coefficient (P=0.05) was also employed to find the correlation between the amount of heart overlapping the PTV and the amount of volume covered by a certain amount of dose (i.e., V5Gy).
Results
Patient characteristics
Relevant patient characteristics are summarized in Table 1. Seventy percent of patients had Stage IIIA disease with the remaining 30% having IIIB disease due to a T4 primary. Sixty percent of patients were men and 40% of patients had left-sided disease. Median PTV size was 435.51 cc (range, 175–1,309.5 cc). Ninety percent of patients had a PTV that overlapped with the esophagus with a median volume of overlap of 10.2 cc (range, 0.4–15.8 cc). For the one patient where the PTV did not overlap with the esophagus, the minimum distance between the two volumes was 0.5 cm. Eighty percent of patients had a PTV that overlapped with the heart with a median overlap volume of 24.1 cc (range, 5.8–71.2 cc). The two patients where the PTV did not overlap with the heart had a minimum distance between the two volumes of 1.4 and 1.7 cm.

Full table
Plan characteristics
In the 3DCRT plans, 3–4 beams were used for all patients (3 beams, n=6, 4 beams, n=4), whilst IMRT plans had 4–5 beams (4 beams, n=7, 5 beams, n=3). IMRT plans had the same (n=3), one extra (n=5) or two extra (n=2) beams compared to 3DCRT. On average IMRT produced 2.3 times more monitor units than 3DCRT. The addition of the heart into the optimization process (IMRT B) resulted in 70% of plans having an increase in monitor units with an average increase of 60.
Impact of utilizing intensity modulated radiotherapy (IMRT) (3DCRT vs. IMRT A)
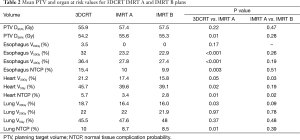
Dosimetric results comparing all plans are summarized in Table 2. IMRT achieved the required PTV minimum coverage in 90% of plans. The one patient with unsatisfactory coverage, had a PTV located adjacent to the spinal canal and IMRT improved the D99% and D95% from 37.4 and 42.6 to 41.3 to 54.3 Gy respectively. Overall, IMRT significantly improved D99% (P=0.01) but produced a similar mean D95% value to the 3DCRT plans.
Full table
Esophagus
IMRT improved the dose to the esophagus at all evaluated dose levels and was able to protect the esophagus from the 60 Gy dose region in all patients. Significant reductions to the mean V50Gy (32% vs. 23.2%, P<0.001) and V40Gy (36.4% vs. 27.8%, P<0.001) levels were seen with the IMRT plans, resulting in a mean NTCP for grade 2 or higher toxicity reducing from 15.4% to 10% (P=0.003).
Lung
IMRT did not compromise lung DVH parameters. The increase in mean V5Gy did not reach statistical significance (P=0.37). NTCP for the lung was reduced in the IMRT plans from 10% to 8.7% (P=0.01). Among the patients in whom the lung V5Gy, V20Gy and V30Gy worsened, the average volume increase was 7% (range, 2.3–11%), 2.2% (range, 0.5–3.6%) and 0.4% (range, 0.1–0.8%) respectively. Of these patients all except one still had a lower lung NTCP. The one exception had an increased NTCP from 6.7% to 7.1%.
Heart
Significant reductions in dose received by the heart were observed in the IMRT plans compared to 3DCRT. Without heart being contoured or considered during optimization, 80% of patients showed a decrease in V5Gy. In this group, the average volume reduction was 8.1% (range, 1.5–19.4%). In the two patients without an improvement, one had a V5Gy of 0% for both plans due to no overlap with the heart, whilst the other had an increase in V5Gy of 1%. Seventy percent of patients demonstrated a decrease in V30Gy with an average improvement of 6.2% (range, 0.1–12.8%). Of the three without an improvement, one had a V30Gy of 0% for both plans whilst the other two had increases of 1.4% and 3.6%. The amount of improvement to heart V5Gy and V30Gy significantly correlated with the percentage of heart overlapping with the PTV (P=0.5) (Figures 1 and 2). IMRT without the heart considered in optimization produced a 2.3% improvement in mean NTCP for cardiac mortality (P=0.01). The three patients with the highest percentage of overlap, 6.4%, 7.2%, 9.9% experienced reduced NTCP of 5.3%, 4.9%, 6.4%.
Impact of considering the heart in optimization (IMRT A vs. IMRT B)
Comparison between the IMRT A and IMRT B plans indicate no significant difference to PTV D95% (57.4 vs. 57.5 Gy, P=0.47), esophagus V40Gy (27.8% vs. 27.4%, P=0.19), V50Gy (23.2% vs. 22.9%, P=0.26), lung V5Gy (47.6% vs.48%, P=0.48), V20Gy (22% vs. 21.9%, P=0.78), V30Gy (16.4% vs. 16%, P=0.09) and heart V5Gy (39.6 vs. 39.1 Gy, P=0.19). IMRT B plans increased the lung V5Gy, V20Gy and V30Gy in 60%, 40% and 20% of patients respectively, by an average of 1.4%, 0.7% and 0.3%. IMRT B plans reduced the heart V30Gy in all patients where the PTV overlapped with the heart, by an average of 2%. Heart V5Gy was reduced in 60% of patients by an average of 1.1%. Of the remaining patients, 20% received no V5Gy in either plan (due to no overlap with the PTV) and 20% experienced increases to the V5Gy by an average of 0.8%. Compared to IMRT A, IMRT B further reduced NTCP for cardiac mortality in all patients, by an average of 0.6% (P=0.02).
Discussion
Our data indicates that IMRT can significantly reduce the dose received by the heart, even when the heart is not incorporated into the inverse optimization process. The majority of patients in our study showed reductions in the volume of heart receiving 5 and 30 Gy, leading to lower NTCP for all cases. These results were achieved purely as a byproduct of using IMRT, when the priority was esophageal sparing without compromising lung doses.
For selected patients, IMRT can provide further sparing to the heart if optimized to do so. This is pertinent to patients with PTVs overlapping the heart. Overall, significant reductions to the volume of heart receiving 30 Gy were achieved, with a further decrease in heart NTCP for all patients where the heart overlapped with the PTV. Importantly these results were achieved without compromising the dose received by the lungs. Prioritizing the heart led to no significant differences to lung V5Gy, V20Gy, V30Gy or lung NTCP. IMRT plans with heart optimized also retained their ability to achieve adequate sparing of the esophagus with similar dosimetric results between both IMRT plans.
Little evidence exists surrounding cardiac toxicity following chemoradiotherapy for NSCLC and current knowledge is largely derived from other diagnoses, mainly Hodgkins disease and breast carcinoma. Long term retrospective data on 2,232 Hodgkins disease patients, indicate radiotherapy is associated with an increased risk of death from ischaemic heart disease, which was most pronounced in doses over 30 Gy (15).The relative risk of acute myocardial infarction death is doubled five years post treatment and continues to increase thereafter with the average time between treatment and death being 10.3 years. A large meta-analysis on early breast cancer patients included 23,500 patients for long-term comparison on radiotherapy versus no radiotherapy outcomes, found radiotherapy significantly increased the risk of mortality from heart disease (Relative risk =1.27) and that this risk remains substantial 10–20 years post treatment (16). Though these risks are concerning it is difficult to interpret how they apply to NSCLC patients who have significant competing risks for death due to smoking related comorbidities and lung cancer itself. RTOG 0617 clearly identified though that heart dose is important, identifying that heart V5Gy and V30Gy are significantly associated with survival (5).
RTOG 0617 has stimulated significant research, with preliminary results presented at the 16th World Conference on Lung Cancer (WCLC). The phase I/II IDEAL-CRT dose escalation trial delivered mean and maximum doses equivalent to 69 and 75.6 Gy in 2 Gy fractions with concurrent chemotherapy to stage II or III NSCLC (17). Investigators have reported a significant link between lower overall survival and the volume of heart receiving 65–75 Gy. They also found the volume of the left ventricle receiving 1–5 Gy to be an indicator for overall survival. Van Der Bijl et al. have retrospectively analyzed 375 patients treated with IMRT to 66 Gy in 24 fractions in a single institution (18). On univariate analysis mean heart dose and volumes receiving 40 Gy or less were strongly associated with overall survival, with V5Gy being most significant. Updated RTOG0617 were also presented at WCLC (19). Further analysis comparing outcomes between 3D-conformal radiotherapy and IMRT were carried out on 482 patients, 47% of whom were treated with IMRT. Despite patients in the IMRT arm having larger PTVs, IMRT exhibited lower lung V20Gy and heart V5Gy, V20Gy and V40Gy and was associated with a lower rate of grade ≥3 pneumonitis (P=0.0653). As Heart V40Gy was associated with poorer overall survival (P<0.001) in the entire population, it appears dose escalation rather than the use of IMRT is what compromised survival in RTOG0617. Furthermore, the RTOG trial protocol recommended the following dosimetric constraints on the heart; 60 Gy to <1/3, 45 Gy to <2/3, 40 Gy to <100%. These are relaxed constraints that were met in all our patients using 3DCRT alone.
The interplay between heart and lung doses and consequential toxicity is complex. Cardiac irradiation is associated with pulmonary dysfunction (20,21). Additionally, the benefit received by reducing the amount of irradiated lung is enhanced by reducing the dose received by the heart (22). This was further investigated in a human cohort by Huang et al. where a retrospective analysis of 209 NSCLC patients treated with radical radiotherapy with or without chemotherapy, revealed heart dosimetric variables to be a significant factor in predicting pneumonitis, particularly when used in conjunction with lung dosimetric variables (23). Similar results were found by Dang et al. (24). Their prospective analysis of 176 NSCLC patients found mean heart dose and the comprehensive dose-volume histogram value of the heart to be strong predictors of ≥ grade 2 and ≥ grade 3 radiation pneumonitis. The results from our study show IMRT can inadvertently produce a significant reduction to the dose received by the heart compared to 3DCRT whilst maintaining acceptable lung tolerance. IMRT is also able to further spare the heart when prioritized in plan optimization. In this study, it was ensured the lung constraints were still achieved when attempts to reduce the heart dose were made. It is most likely though, that further sparing of the heart could be achieved at the expense of increasing doses to the lungs. The question then arises; what is the optimal compromise between these two organs to achieve the best toxicity and survival outcomes? The answer should be further explored in future research.
Conclusions
Based on current evidence, cardiac sparing should be a priority when delivering chemoradiotherapy for locally advanced NSCLC. Utilizing IMRT appears to achieve this goal even when the heart volume is not incorporated in the optimization process. Further research into the optimal combination of heart and lung DVH parameters is justified.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S.
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Palma DA, Senan S, Oberije C, et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;87:690-6. [Crossref] [PubMed]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003;57:875-90. [Crossref] [PubMed]
- Al-Halabi H, Paetzold P, Sharp GC, et al. A Contralateral Esophagus-Sparing Technique to Limit Severe Esophagitis Associated With Concurrent High-Dose Radiation and Chemotherapy in Patients With Thoracic Malignancies. Int J Radiat Oncol Biol Phys 2015;92:803-10. [Crossref] [PubMed]
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301-10. [Crossref] [PubMed]
- International Commission on Radiation Units and Measurement. ICRU Report No. 50. Prescribing, recording and reporting photon beam therapy. Washington DC, 1993.
- International Commission on Radiation Units and Measurement. Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). Journal of the ICRU 2010;10.
- Uzan J, Nahum AE. Radiobiologically guided optimisation of the prescription dose and fractionation scheme in radiotherapy using BioSuite. Br J Radiol 2012;85:1279-86. [Crossref] [PubMed]
- De Jaeger K, Hoogeman MS, Engelsman M, et al. Incorporating an improved dose-calculation algorithm in conformal radiotherapy of lung cancer: re-evaluation of dose in normal lung tissue. Radiother Oncol 2003;69:1-10. [Crossref] [PubMed]
- Belderbos J, Heemsbergen W, Hoogeman M, et al. Acute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol 2005;75:157-64. [Crossref] [PubMed]
- Eriksson F, Gagliardi G, Liedberg A, et al. Long-term cardiac mortality following radiation therapy for Hodgkin's disease: analysis with the relative seriality model. Radiother Oncol 2000;55:153-62. [Crossref] [PubMed]
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949-55. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Vivekanandan S, Counsell N, Parsons E, et al. Dose-Escalated Radiotherapy for NSCLC: Heart Doses versus Survival in IDEAL-CRT. Journal of Thoracic Oncology 2015;10:S384.
- Van Der Bijl E, Rossi MM, Peulen H, et al. Heart Dose Is Associated with Shorter Overall Survival for Patients Treated with Chemo-Radiation for Locally Advanced NSCLC. J Thorac Oncol 2015;10:S384.
- Chun SG, Hu C, Choy H, et al. Outcomes of Intensity Modulated and 3D-Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer in NRG Oncology/RTOG 0617. J Thorac Oncol 2015;10:S213.
- Novakova-Jiresova A, van Luijk P, van Goor H, et al. Pulmonary radiation injury: identification of risk factors associated with regional hypersensitivity. Cancer Res 2005;65:3568-76. [Crossref] [PubMed]
- van Luijk P, Novakova-Jiresova A, Faber H, et al. Radiation damage to the heart enhances early radiation-induced lung function loss. Cancer Res 2005;65:6509-11. [Crossref] [PubMed]
- van Luijk P, Faber H, Meertens H, et al. The impact of heart irradiation on dose-volume effects in the rat lung. Int J Radiat Oncol Biol Phys 2007;69:552-9. [Crossref] [PubMed]
- Huang EX, Hope AJ, Lindsay PE, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol 2011;50:51-60. [Crossref] [PubMed]
- Dang J, Li G, Ma L, et al. Predictors of grade ≥ 2 and grade ≥ 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol 2013;52:1175-80. [Crossref] [PubMed]


