Prevalence of neutralizing antibodies to common respiratory viruses in intravenous immunoglobulin and in healthy donors in southern China
Introduction
Acute respiratory infections (ARIs) are a leading cause of death among children under the age of five, killing more than four million people per year and accounting for over 8% of the global burden of disease. The infections often result in pneumonia, which is the single largest infectious cause of death in children worldwide and killed an estimated 935,000 children under the age of five in 2013 (1). ARIs can be caused by viruses, bacteria, or fungi. The availability of improved diagnostic tools has led to the identification in recent years of viruses as an increasingly frequent cause of pneumonia (2). Previous work from our group and others found that respiratory syncytial virus (RSV), seasonal influenza A (InfA), enterovirus (EV), and adenovirus (Ad) were the predominant pathogens in children with ARI (3-6). Adenovirus type 3 (Ad3) and Ad7 were the most prevalent serotypes of Ad that caused ARI (7,8), but in recent years adenovirus type 55 (Ad55) has also caused severe pneumonia and death in children and adults worldwide (9,10).
There is no effective medicine for most severe viral infections and general supportive care is currently the only clinical option (11), so new treatments for these infections are desperately needed. One potential therapeutic option is passive immunotherapy with convalescent plasma or hyperimmune intravenous immunoglobulin (H-IVIG) (12). Previous work has suggested that this approach might be an effective treatment for infections with Spanish InfA (H1N1), avian InfA (H5N1), 2009 pandemic InfA (H1N1), SARS coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV), among others (12-18). However, few studies have been reported using this approach for the most prevalent viral pathogens. Infections with these common viruses have a lower fatality rate than those caused by the viruses listed above, but they cause a large number of severe ARIs (SARIs) and even deaths worldwide every year.
For passive immunotherapy, it is important to screen convalescent plasma and prepare H-IVIG from convalescent serum with high titers of neutralizing antibodies (NAbs) (19-22). Intravenous immunoglobulin (IVIG), which is produced from a large pool of healthy volunteers with unknown NAb titers, has been used clinically for preventing and treating bacterial and virus infections (23-27). And H-IVIG from vaccine-immunized donors has been used clinically for preventing and treating specific virus infections, such as rabiesvirus and HBV. Given the high infection rate in childhood of viruses causing ARIs, there may be high titers of NAbs against these pathogens in the adult population and, therefore, in IVIG. So healthy blood volunteers may be a suitable source of convalescent plasma. However, up to now, only a few reports have described a neutralizing activity against specific respiratory viruses in support of this hypothesis (28-33). Therefore, in the present study we has investigated the prevalence and serum titers of NAbs against the main viruses that cause ARIs, including RSV, seasonal InfA, EV [enterovirus 71 (EV71) and coxsackievirus A16 (CA16)] and Ad (Ad3), as well as those against a rare virus Ad55 which has caused several epidemic outbreaks in recent years, in healthy adults in southern China. We also evaluated the NAb titers against these viruses in commercially available IVIG to discuss the need for preparing H-IVIG from convalescent serum.
Methods
Human serum samples
Serum samples from 96 healthy donors were collected at random in 2014 by Dongguan Children’s Hospital in Dongguan, southern China. Donors ranged in age from 20 to 49 years old with a sex ratio of 1:1 without any other patient identifier. This research involving human participants was approved by the Ethics Committee of the Affiliated First Hospital of Guangzhou Medical University, and informed consent was obtained from each of the volunteers.
Human immunoglobulin for intravenous injection (IVIG)
A total of ten bottles of human immunoglobulin (pH4) for intravenous injection with an IgG protein concentration of 5% (50 mg/mL; IVIG) from five production lots were used in this study. They were produced by Guangdong Shuanglin Bio-Pharmacy Co., Ltd. between January 2014 and October 2014 from plasma collected from healthy donors. The IVIG was diluted with Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, China) before use.
Virus neutralization assays
The following virus strains were used for neutralization assays: human Ad3 GZ01 strain (Genebank No. DQ099432), enterovirus type 71 GZ08 strain (Genebank No. FJ360545), coxsackie virus A16 GZ08 strain (Genebank No. FJ198212.1), human respiratory syncytial virus A strain Long (RSV) from ATCC (Genebank No. AY911262.1), InfA strain PR8 (A/PR8/34, H1N1) from ATCC, human Ad55 Shanxi-Y16 strain (GenBank No. KF911353.1) which was kindly provided by Lin Chen of Guangzhou Medical University (Guangzhou, China).
The NAbs against the respiratory viruses in both human serum samples and commercially available IVIG samples were quantitatively detected by standard in vitro microneutralization tests with appropriate cell types. In brief, human embryonic kidney (HEK) 293 cells (for HAds), Vero cells (for EV71 and CA16), HEp-2 cells (for RSV), and MDCK cells (for InfA) were seeded at a density of 2×104 cells per well in 96-well plates and cultured for 24 hours. Concurrently, 100 50% tissue culture infectious dose (TCID50) of the viruses were mixed with equal volumes of 2-fold serially-diluted human serum or IVIG at 37 °C for 1 hour. Then, the mixtures were adsorbed onto the corresponding cells and incubated for 48 hours. Titers from triplicate wells were read as the highest dilution of sera that inhibited virus growth without visible cytopathic effect (CPE).
Statistical analyses
Comparisons between the seroprevalence of different groups were evaluated using Pearson’s χ2 for binary variables (with Fisher’s exact method when appropriate), and statistical analyses were computed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). For all analyses, P values of less than 0.05 were regarded as significant.
Results
Seroprevalence in the healthy adult population of NAbs against respiratory viruses
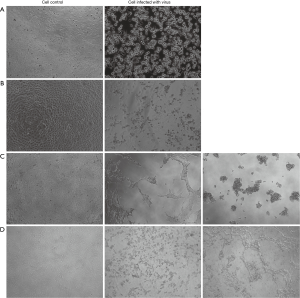
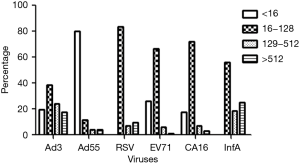
First, we wanted to evaluate the seroprevalence in healthy adults of NAbs against common respiratory viruses. As shown in Figure 1, after incubation for 48 hr, the typic CPE could be seen in cells infected with RSV, InfA, Ad3, Ad55, EV71 or CA16. We collected 96 serum samples from healthy donors and performed microneutralization assays to measure the titers of NAbs against several respiratory viruses. As shown in Figure 2, only to Ad55, a high proportion of samples from healthy adult donors were negative for NAbs (>16); to the other five viruses the highest proportion of samples has NAb at titer 16–128. As shown in Table 1, 80.21%, 19.79%, 100.00%, 73.96%, 82.29%, and 100.00% of the samples were positive (NAb titers ≥16) for Ad3, Ad55, RSV, EV71, CA16, and InfA, respectively; and 41.67%, 8.34%, 16.67%, 7.29%, 10.42%, and 43.75% of the samples had notably high NAb titers (>128) against Ad3, Ad55, RSV, EV71, CA16, and InfA, respectively. All of the samples were positive for RSV and InfA NAbs. Many samples had high NAb titers (>512) for RSV (9.38%), InfA (25.00%) and Ad3 (17.71%) and several samples had extremely high NAb titers (>2,000) for Ad3. However, a high proportion of samples, 80 (83.33%), 64 (66.67%), and 69 (71.88%) had low but significant NAb titers [16–128] for RSV, EV71, and CA16, respectively. Only 19.79% of the samples were positive for Ad55 NAbs, but some of these (4.17%) had high (>512) titers. The mean of NAbs titer were 221, 216, 158, 53, 61, 70 to InfA, Ad3, RSV, Ad55, EV71 and CA16, respectively.
Full table
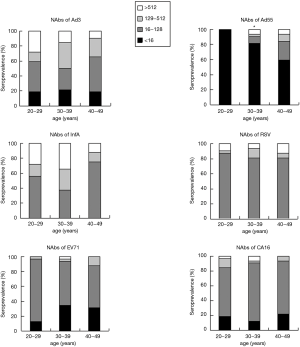
Further comprehensive analyses were performed and frequency histograms were drawn to illustrate the impact of age on respiratory virus infection. The percentage of samples from age groups of 20–29, 30–39, and 40–49-year-old that were positive for Ad55 NAbs were 0%, 19.75%, and 40.625%, respectively, as demonstrated in Table 2. The percentage of samples that were positive for Ad55 NAbs (titers >16) increased with the age of the donors (P<0.01). For the other tested viruses, there were no significant differences in the proportions of samples positive for NAbs between age groups (Figure 3). Additionally, there were not any significant differences in the proportions of NAb titers between males and females for any of the tested viruses (Table 3).

Full table

Full table
A total of 36 (37.5%) samples were positive for NAbs against all five of the most common viruses that were tested: RSV, EV71, CA16, Ad3, and InfA. Eighty (83.33%) samples were positive for NAbs against at least four of these five viruses, and 92 (95.83%) samples against at least three of them.
NAb titers of IVIG
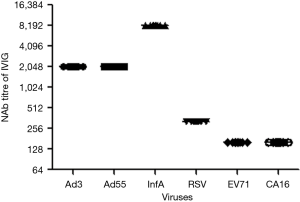
Next, we wanted to assess the NAb titers against the same common respiratory viruses in commercially available IVIG. A total of ten bottles of five human immunoglobulin lots (pH4) intended for intravenous injection were assessed. These samples had an IgG protein concentration of 5% (50 mg/mL). The IVIG were then diluted with DMEM, and the NAb titers were detected by standard microneutralization tests. As shown in Figure 4, all of the IVIG from different production lots had the same titers against the same viruses. IVIG had extremely high NAb titers to InfA [8192] and Ad3 [2048]. It is striking that IVIG also had a high NAb titer to Ad55 [2048]. However, IVIG had lower NAb titers to RSV [320], EV71 [160], and CA16 [160], which corresponds with the lower proportions of high NAb titer (>128) samples that we found in healthy donors (Figure 2).
Discussion
It may be possible to treat ARI using convalescent plasma or serum IVIG containing high titers of NAbs against specific pathogens. Passive immunotherapy, which mainly uses convalescent plasma or convalescent serum, and H-IVIG prepared from convalescent sera, has been suggested as a potentially effective method to prevent and treat severe viral infection (12). This method has been used for treating ARI caused by H5N1, H1N1, SARS-CoV, and MERS-CoV (11-16). However, ARIs in children are mainly caused by RSV, InfA, EV, and Ad (3). Therefore, passive immunotherapy should be further studied as a potential treatment for ARI in children. For passive immunotherapy, the screening and preparation of convalescent plasma or serum is the main difficulty (19). In general, convalescent plasma is prepared from donors who are diagnosed patients or volunteers who have been vaccinated with high titers of specific antibodies. H-IVIG is then produced from a mixture of convalescent plasmas from different individuals (16). There are few reports about the seroprevalence of NAb titers against common respiratory viruses in healthy adult donors (29-31). In this study, we piloted a successful attempt to screen plasma with high titers of NAb against common respiratory viruses from healthy adult donors, and we found that the commercial IVIG contained high titers of NAbs against RSV, InfA, EV, and Ad.
A high proportion of healthy adult donors were found to have NAbs against these common respiratory viruses (Table 1). The high rates of vaccination against and infection with InfA may explain our finding that the highest proportion of NAb positive samples was for this virus and that many of the positive samples had high NAb titers against InfA. Only the seasonal InfA vaccine is used in China; however, no vaccines are used here for Ad, RSV, EV71, or CA16. Our finding that a high percentage of the samples were positive for these viruses demonstrates the high infection rate of these viruses in the Chinese population. A sizable proportion of the samples had high NAb titers (>512) for InfA (25%) and Ad3 (17.71%). Although they were less abundant, some samples also had high NAb titers against RSV (9.38%), EV71 (1.04%), CA16 (3.13%), and Ad55 (4.17%). These results suggest that healthy blood volunteers may be a suitable source for convalescent plasma for use in treating SARIs and preparing blood banks against these specific viruses (20). Clinically, SARIs are still common in southern China, especially in children and immunodeficient patients. Currently, these cases are sometimes treated using plasma with undetermined NAb titers as general supportive care. In these cases it may be more effective to use plasma with a high NAb titer.
With these resources, the relationship between the NAb titer against respiratory viruses and the therapeutic effect could be studied. Additionally, the blood with high titers of NAbs against respiratory viruses also could be used to specifically screen for human neutralizing monoclonal antibodies against individual viruses. In this study, we used the InfA standard strain A/PR8/34 (H1N1), which was isolated over 80 years ago, to evaluate the prevalence of NAbs, and we found that 25% of the serum samples had high NAb titers against H1N1. This result could be explained by the presence of cross-reactive NAbs from natural infection with H1N1 (28,31,34-36). However, due to the high variability in influenza virus antigenicity, it might be more appropriate to screen plasma using recent epidemic influenza strains.
The percentage of samples that were positive for NAbs against Ad55 (titers >16) increased with age (P<0.01), as demonstrated in Figure 3 and Table 2. For NAbs against other viruses, there were no significant differences in the positive proportions between age groups 20–29, 30–39, or 40–49 years. This trend agrees with previous work showing that Ad55 are often detected in the adult population in contrast with EV71, RSV, CA16, and Ad3, which are mainly detected in children (5-9). There were not any significant differences in the proportions of positive samples or in the titers of NAbs between males and females for any of the tested viruses (Table 3). Based on these results, blood from male or female 20–49-year-old volunteers can be used for screening NAbs against these viruses.
H-IVIG is prepared from the convalescent plasma of patients with a specific pathogen infection. Unlike H-IVIG, standard IVIG is produced in the blood of healthy donors and has been widely used clinically for preventing infection. IVIG has also been successfully used for treating CMV infection and parvovirus B19 infection (26,27). Only H-IVIG has been used for treating infections with SARS-CoV, MERS-CoV and H5N1, which is likely because of the low infection rates of these viruses in the population (12). The high titers of NAbs against Ad3, and InfA (Figure 4) that we found in samples from healthy donors suggest that IVIG might be useful for treating SARIs caused by these viruses. We found that the NAb titers in IVIG are not as high against RSV [320], EV71 [160], and CA16 [160] as against InfA [8192] and Ad3 [2048], Ad55 [2048] (Figure 4); therefore, high-dose IVIG may be needed for treating infections caused by these viruses. To our knowledge, there have been no studies testing the use of IVIG to treat infections with these viruses, but our results suggest that this treatment should be investigated. However, high-dose IVIG used in children may cause side effect, such as allergic reactions. And commercial IVIG is expensive. It is easy to understand that H-IVIG prepared only from sera with high titer NAb against specific virus would have a much higher titer NAb against this virus, and then could be used at a low-dose. Due to the presences of high NAbs against these viruses in adults, H-IVIG could be prepared from screened sera with high titer NAbs as personalized medicine which may be more economical and effective for treating specific respiratory virus infection.
Ad55 is a new type of Ad, which was recombined from Ad14 and Ad11, and it has caused severe infections in China and other regions since 2006 (7-10). It is interesting to find that only a low proportion of samples from adult donors were positive for NAbs to Ad55, but that commercially available IVIG had a high NAb titer against this virus. These findings may be due to the fact that the commercially produced IVIG was prepared from healthy donors who came from a larger area. The low proportion of samples from our donors that were positive for NAbs against Ad55 suggests that there may be little protection in the population of southern China and indicates that this area should be frequently surveyed for Ad55 infection. The high titers of NAb against Ad55 in IVIG suggest that IVIG may be potentially effective as an early treatment for Ad55 infection and this possibility should be investigated further.
Conclusions
Convalescent plasma with high NAb titers against common respiratory viruses (Ad3, Ad55, InfA, RSV, EV71 and CA16) can be screened from healthy blood volunteers to establish blood banks for treating SARIs. Additionally, commercial IVIG had high titer NAbs against Ad3, Ad55 and InfA, but low titer NAbs against RSV, EV71 and CA16, which indicates that it may be necessary to prepare H-IVIG from sera with high titer NAbs for treating these specific virus infections. This research provides useful data for further developing passive immunotherapy against SARI.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (NSFC 31200132 and 31570163), Science and Technology Projects of Guangzhou (201508020252, 201504010032), State Major Infectious Disease Research Program (China Central Government, 2012ZX10004-213).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization (WHO). Acute respiratory infections. Available online: http://www.who.int/topics/respiratory_tract_diseases/en/
- Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet 2011;377:1264-75. [PubMed]
- Liu WK, Liu Q. Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study. PLoS One 2014;9:e96674. [PubMed]
- Williams BG, Gouws E, Boschi-Pinto C, et al. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002;2:25-32. [PubMed]
- Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA 2010;303:2051-7. [PubMed]
- Marcone DN, Ellis A, Videla C, et al. Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos Aires, Argentina. Pediatr Infect Dis J 2013;32:e105-10. [PubMed]
- Sandkovsky U, Vargas L, Florescu DF. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep 2014;16:416. [PubMed]
- Deng J, Qian Y, Zhao LQ, et al. Identification and typing of adenovirus from acute respiratory infections in pediatric patients in Beijing from 2003 to 2012. Bing Du Xue Bao 2013;29:615-20. [PubMed]
- Lu QB, Tong YG, Wo Y, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respir Viruses 2014;8:302-8. [PubMed]
- Cao B, Huang GH, Pu ZH, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 2014;145:79-86. [PubMed]
- Kelesidis T, Mastoris I, Metsini A, et al. How to approach and treat viral infections in ICU patients. BMC Infect Dis 2014;14:321. [PubMed]
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015;211:80-90. [PubMed]
- Luke TC, Casadevall A, Watowich SJ, et al. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med 2010;38:e66-73. [PubMed]
- Hui DS, Lee N, Chan PK. Adjunctive therapies and immunomodulatory agents in the management of severe influenza. Antiviral Res 2013;98:410-6. [PubMed]
- Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007;357:1450-1. [PubMed]
- Hung IF, To KK, Lee CK, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013;144:464-73. [PubMed]
- Hohenadl C, Wodal W, Kerschbaum A, et al. Hyperimmune intravenous immunoglobulin containing high titers of pandemic H1N1 hemagglutinin and neuraminidase antibodies provides dose-dependent protection against lethal virus challenge in SCID mice. Virol J 2014;11:70. [PubMed]
- Park UJ, Hyun SK, Kim HT, et al. Successful treatment of disseminated adenovirus infection with ribavirin and intravenous immunoglobulin in an adult renal transplant recipient: a case report. Transplant Proc 2015;47:791-3. [PubMed]
- Wong HK, Lee CK, Hung IF, et al. Practical limitations of convalescent plasma collection: a case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion 2010;50:1967-71. [PubMed]
- Leider JP, Brunker PA, Ness PM. Convalescent transfusion for pandemic influenza: preparing blood banks for a new plasma product? Transfusion 2010;50:1384-98. [PubMed]
- Parry RP, Tettmar KI, Hoschler K, et al. Strategies for screening blood donors to source convalescent H1N1v plasma for intervention therapy. Vox Sang 2012;103:107-12. [PubMed]
- Zhang Z, Xie YW, Hong J, et al. Purification of severe acute respiratory syndrome hyperimmune globulins for intravenous injection from convalescent plasma. Transfusion 2005;45:1160-4. [PubMed]
- Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. N Engl J Med 1988;319:902-7. [PubMed]
- Chapel HM, Lee M, Hargreaves R, et al. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma. Lancet 1994;343:1059-63. [PubMed]
- Magny JF, Bremard-Oury C, Brault D, et al. Intravenous immunoglobulin therapy for prevention of infection in high-risk premature infants: report of a multicenter, double-blind study. Pediatrics 1991;88:437-43. [PubMed]
- Bowden RA, Fisher LD, Rogers K, et al. Cytomegalovirus (CMV)-specific intravenous immunoglobulin for the prevention of primary CMV infection and disease after marrow transplant. J Infect Dis 1991;164:483-7. [PubMed]
- Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis 2013;56:968-77. [PubMed]
- Hong DK, Tremoulet AH, Burns JC, et al. Cross-reactive neutralizing antibody against pandemic 2009 H1N1 influenza a virus in intravenous immunoglobulin preparations. Pediatr Infect Dis J 2011;30:67-9. [PubMed]
- Kubota-Koketsu R, Yunoki M, Okuno Y, et al. Significant neutralizing activities against H2N2 influenza A viruses in human intravenous immunoglobulin lots manufactured from 1993 to 2010. Biologics 2012;6:245-7. [PubMed]
- Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945-52. [PubMed]
- Henry Dunand CJ, Leon PE, Kaur K, et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest 2015;125:1255-68. [PubMed]
- Zhang S, Huang W, Zhou X, et al. Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J Med Virol 2013;85:1077-84. [PubMed]
- Liu X, Liu Y, Zhang Y, et al. Pre-existing immunity with high neutralizing activity to 2009 pandemic H1N1 influenza virus in Shanghai population. PLoS One 2013;8:e58810. [PubMed]
- Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012;109:9047-52. [PubMed]
- Miller MS, Gardner TJ, Krammer F, et al. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 2013;5:198ra107.
- Xu R, Ekiert DC, Krause JC, et al. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010;328:357-60. [PubMed]


