Malignant transformation of alveolar adenoma to papillary adenocarcinoma: a case report
Introduction
Alveolar adenoma (AA) is a rare lung neoplasm and typically presents as a peripherally located, well-circumscribed, solitary nodule. AA is considered a benign neoplasm, with no cases of recurrence having been described in the literature. Here, we present a case of AA comprising a malignant component.
Case representation
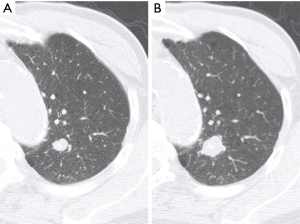
An 83-year-old asymptomatic man was referred to our department for evaluation of a solitary pulmonary nodule found upon radiography. He was an ex-smoker (40 pack-years). Chest computed tomography revealed a well-circumscribed and calcified nodule (diameter: 11 mm) in the apical posterior segment of the left upper lobe (S1+2). Although the nodule was suspected to be granuloma, its diameter increased to 18 mm in 15 months interval (Figure 1). Fluorine-18-fluorodeoxyglucose positron emission tomography showed minor uptake in the left upper lobe nodule, with a standardized uptake value of 1.4. Transbronchial biopsy yielded a pathological diagnosis of adenocarcinoma (AC). Therefore, considering his low pulmonary function and the localization of the nodule, left S1+2 segmentectomy was performed under video-assisted thoracoscopic surgery.
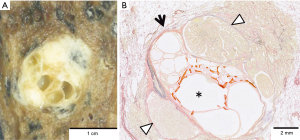
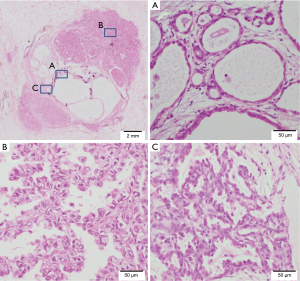
Macroscopically, the tumor was a well-circumscribed, apparently solitary mass. The cut surface was pale tan-colored and revealed multi-cystic lesions centrally (Figure 2A). Microscopically, the tumor consisted of two histologically distinct areas—a central multi-cystic area and a surrounding area (Figure 2B). The central area consisted of various-sized cystic structures that showed expansive growth and were lined with a single layer of cuboidal epithelial cells without cytological atypia (Figure 3A). The interstitial component contained delicate collagen fibers with metaplastic ossification, without elastic fibers, suggesting a trace of alveolar structure. The epithelial component was immunohistochemically positive for thyroid transcription factor-1 (TTF-1), surfactant apoprotein A, Napsin A, and carcinoembryonic antigen, and negative for tumor suppressor p53. These findings indicated AA. The surrounding area was determined as papillary predominant AC with bronchiolar invasion and intraluminal growth (Figure 3B). The tumor cells were immunohistochemically positive for TTF-1, surfactant apoprotein A, Napsin A, carcinoembryonic antigen, and p53. Both the AA and AC were negative for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) protein expression. The borderline lesion between the AA and AC showed intermediate cytological atypia with low papillary growth of the tumor cells in the same stroma as the AA (Figure 3C). The mindbomb homolog-1 (MIB-1) index was 1% in the AA, 6% in the borderline lesion, and 14% in the AC. Based on these findings; AC arising from AA was diagnosed. No recurrence was reported during the postoperative follow-up (48 months).
Discussion
AA is a very rare lung neoplasm first described in 1986 by Yousem and Hochholzer (1), and there are currently only 40 English-language reports on AA (2). Patients with AA are usually asymptomatic and middle-aged, and there is a slight female predominance. In most cases, this tumor is incidentally detected as a peripheral or subpleural coin lesion on chest radiography, and complete resection has been shown to offer a good prognosis without recurrence (2).
The origin of this neoplasm is still unknown; however, based on its histological findings, most authors agree that the epithelial component of AA is derived from type II pneumocytes (3). The major histological features include a well-circumscribed non-encapsulated cystic mass comprising cuboidal epithelial cells immunohistochemically positive for TTF-1, without atypia, and surrounded by a myxoid and collagenous interstitium (4). AA should be distinguished from solitary pulmonary nodules, such as sclerosing hemangioma, lymphangioma, atypical adenomatous hyperplasia (AAH), and AC with lepidic growth pattern (previously called bronchioloalveolar carcinoma) (3). In particular, AAH, which is considered a precursor lesion of AC of the lung, shows atypical bronchioloalveolar proliferation, while AC with a lepidic growth pattern demonstrates cytological atypia and a lepidic infiltrative growth pattern.
There are no reports describing malignancy of AA. Bhavsar et al. reported a case of microscopic AA coexisting with, but clearly separated from, lung carcinoma (5). In contrast, our case involved an AA surrounded by AC. Moreover, the borderline area showed transitional patterns of the morphological features and proliferation. The MIB-1 index, which reflects cellular proliferative activity and malignancy in lung AC (6), is usually less than 1% in AA (7). However, our case demonstrated a gradual increase of the MIB-1 index from the AA to the invasive AC. Taken together, these findings strongly suggest malignant transformation of AA, rather than collision of two separate tumors. We need to keep in mind a future possibility of malignant transformation of AA or a coexisting lung carcinoma, especially when the lung tumor is diagnosed as AA by a biopsy specimen.
Nowadays, analysis of driver mutations in lung cancer, such as EGFR and ALK, is employed for understanding individual genetic characteristics and deciding the appropriate molecular targeted therapy. The AA in the present case was negative for EGFR mutation and ALK protein expression; however, the genetic features of AA have not yet been thoroughly analyzed, owing to its rarity (8). Thus, further genetic analyses, as well as immunohistochemical studies, are needed to clarify its nature.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The study participant provided informed consent for the findings of this case to be published.
References
- Yousem SA, Hochholzer L. Alveolar adenoma. Hum Pathol 1986;17:1066-71. [Crossref] [PubMed]
- Wang X, Li WQ, Yan HZ, et al. Alveolar adenoma combined with multifocal cysts: case report and literature review. J Int Med Res 2013;41:895-906. [Crossref] [PubMed]
- Sak SD, Koseoglu RD, Demirag F, et al. Alveolar adenoma of the lung. Immunohistochemical and flow cytometric characteristics of two new cases and a review of the literature. APMIS 2007;115:1443-9. [Crossref] [PubMed]
- Beasley MB, Husain AN, Travis WD. Alveolar adenoma. In: Travis WD, Brambilla E, Burke AP, et al., editors. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2015:112-3.
- Bhavsar T, Uppal G, Travaline JM, et al. An unusual case of a microscopic alveolar adenoma coexisting with lung carcinoma: a case report and review of the literature. J Med Case Rep 2011;5:187. [Crossref] [PubMed]
- Kawatsu Y, Kitada S, Uramoto H, et al. The combination of strong expression of ZNF143 and high MIB-1 labelling index independently predicts shorter disease-specific survival in lung adenocarcinoma. Br J Cancer 2014;110:2583-92. [Crossref] [PubMed]
- Nakamura H, Adachi Y, Arai T, et al. A small alveolar adenoma resected by thoracoscopic surgery. Ann Thorac Surg 2009;87:956-7. [Crossref] [PubMed]
- Roque L, Oliveira P, Martins C, et al. A nonbalanced translocation (10;16) demonstrated by FISH analysis in a case of alveolar adenoma of the lung. Cancer Genet Cytogenet 1996;89:34-7. [Crossref] [PubMed]


