Bioresorbable vascular scaffolds in patients with acute myocardial infarction: a new step forward to optimized reperfusion?
Introduction
Major advances have occurred during the last decade in the treatment of patients presenting with acute myocardial infarction (MI) (1). These include logistic improvements leading to the organization of efficient network programs that enable timely and optimal primary angioplasty procedures, the advent of novel antithrombotic regimens and the use of new-generation drug-eluting stents (DES) (1,2). Patients with ST-segment elevation MI (STEMI) are associated with a complex underlying coronary substrate leading to a higher rate of restenosis but also to a higher risk for stent thrombosis. The controversy on the value of first-generation DES versus conventional bare-metal stents (BMS) in these patients was maintained for some years (1). Although DES were able to significantly reduce the restenosis rate the possibility of increasing the risk of late and very late stent thrombosis was a cause of concern (1). Suboptimal stent implantation (mainly undersizing) secondary to difficulties to accurately ascertain the true vessel size in the presence of vasospasm secondary to sympathetic activation and to the existence of a large residual thrombus burden, were implicated in the appearance of adverse long-term clinical events. Accordingly, careful thromboaspiration was advocated to optimize acute procedural results and to prevent the occurrence of late acquired malapposition resulting from the disappearance of the residual thrombus entrapped behind the stent (1,2). However, the widespread systematic utilization of manual thrombus aspiration during routine primary angioplasty procedures has been recently halted in the light of the negative results of 2 large controlled trials of routine thromboaspiration in STEMI powered for major clinical events (1,3). Moreover, the delayed healing and the potential toxic effects on the vessel wall leading to positive remodeling and late acquired malapposition occasionally seen with first generation DES (4), were additional issues of concern explaining the delayed widespread adoption of DES in STEMI patients (1). Nevertheless, novel generation DES have proved to be not only more effective but also safer than first generation DES (5). Indeed, studies demonstrated that rates of stent thrombosis were even lower with second-generation DES than with BMS (6). Importantly, in patients with STEMI, the EXAMINATION randomized clinical trial demonstrated that everolimus-DES (EES) were associated with a reduced rate of stent thrombosis compared with BMS (7). In this study EES also significantly reduced the rates of target-lesion revascularization (7). Moreover, the 5-year results of this randomized trial (8) confirmed that the sustained clinical efficacy of EES in STEMI patients may translate into a survival improvement.
Attractiveness of bioresorbable vascular scaffolds (BVS) in STEMI
BVS represent a disruptive technology leading to a new revolution in interventional cardiology (1,9,10). Current generation BVS provide nearly the same scaffolding properties than metallic stents ensuring optimal acute anatomic results. However, to obtain a similar radial force and prevent acute recoil currently available BVS have thicker struts (150 µm). Actually, BVS maintain better conformability than new-generation metallic DES. However, the crossing profile and device pushability and deliverability remain a limitation of these devices specially in tortuous and calcified vessels (9,10). Likewise, as the dilation range is rather narrow optimal scaffold sizing remains of paramount importance to avoid fracture due to over-dilation. BVS elute the antiproliferative drug with a similar efficacy to metallic DES (9,10). In fact, the amount of everolimus eluded is larger from BVS than from EES. In favourable clinical and anatomic scenarios the long-term clinical and angiographic results of BVS appear to be comparable to those obtained with DES (9,10). Accumulating evidence also suggest the long-term clinical safety and efficacy of BVS used in more complex clinical and anatomic settings (11). Nevertheless, in a “real world” routine clinical practice some studies with an “all-comers” design have suggested the possibility of increased risk of acute and subacute thrombosis associated with the use of BVS (12). The technical subtleties and nuances associated with the delivery and implantation of these early generation scaffolds (thicker struts and less flexible devices) have been implicated. Attention to adequate predilation, accurate sizing and optimal postdilation have been suggested to prevent these potential problems, especially in patients with complex lesions. The “soft” lesions that characterize STEMI patients (ruptured thin-cap fibroatheromas with a large necrotic core, positive vessel remodelling and large intraluminal thrombus) may provide an ideal substrate for BVS implantation. Some investigators suggest a potential benefit of slight scaffold oversizing in these patients. However, the risk of no reflow phenomenon could be higher when aggressive post-dilation is systematically performed. The higher strut-to-vessel ratio that characterizes current BVS as compared with second-generation DES might facilitate the entrapment of the residual thrombus (“snow racket effect”) and prevent silent distal embolization or clinically evident no reflow phenomena. However, the potential risks of BVS implantation in a highly thrombogenic milieu, as in STEMI patients, should be critically assessed. In these patients, the use of thromboaspiration and novel potent antiplatelet agents (prasugrel or ticagrelor) before BVS implantation is, therefore, particularly appealing.
The beauty of BVS is simply that they eventually completely disappear from the vessel wall after serving their function (9,10). Polymeric scaffolds consist of polylactide (a mixture of crystalline and amorphous poly-L-lactic acid) that is degraded to lactic acid that, in turn, is hydrolyzed to CO2 and H2O via the Krebs cycle (9,10). The absence of a permanent metallic cage and durable polymer coatings on the vessel wall is very attractive indeed. BVS may overcome some shortcomings associated with permanent metallic jailing of side-branches and the “freezing” of the vessel wall preventing remodelling phenomena able to compensate for plaque growth or even promote lumen enlargement. Furthermore, BVS dissipate concerns on the risks associated with delayed healing and endothelialization of the stent struts and those related with very late malapposition (5). Preliminary studies already suggest that coronary vasomotion and normal vessel wall physiology are restored at long-term follow-up after BVS implantation (13). In addition, the possibility of a significant reduction in the underlying plaque burden associated with late lumen enlargement has been recently suggested (14). Finally, in patients with acute coronary syndrome treated with BVS the late healing process appears to be associated with the development of a novel neointimal layer or “thick cap” that covers, seals and potentially “stabilizes” the underlying guilty pathologic substrate (15). All the advantages associated with this phenomenon known as late “vessel restoration” could be of particular value in STEMI patients. In addition, STEMI patients tend to be younger and have proximal non-calcified culprit plaques with less extensive disease and, theoretically speaking, may particularly benefit from not having a long-life permanent rigid metallic structure on their coronary arteries.
Typical examples of BVS results in STEMI patients are presented in Figures 1-4.

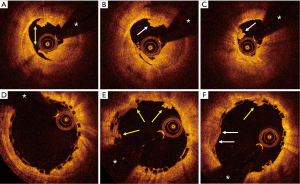
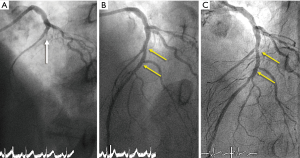
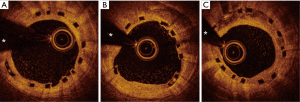
Studies addressing the value of bioresorbable vascular scaffolds (BVS) in STEMI
Several preliminary observational studies demonstrated the safety and feasibility of BVS implantation in STEMI patients (16-20). PRAGUE 19 (19) was a prospective registry where consecutive patients with STEMI were treated with BVS as a default strategy. Of 142 patients treated with primary angioplasty 41 (29%) fulfilled criteria for BVS implantation that was successful in 98% of cases. The event-free survival for patients treated with BVS was 95% vs. 93% for a control group of STEMI patients treated with metallic stents. In a subsequent report from these investigators (20) computed tomographic angiography was performed after 1 year in 59 patients showing a binary restenosis rate of only 2%. Most of these early observational studies, however, were limited by the lack of a control group, small sample size and short-term follow-up. More recently Cortese et al. (18) analyzed 563 patients with STEMI included in a large Italian registry; of these, 122 received BVS and 441 EES. At a median of 220 days, no significant differences were observed in terms of patient-oriented clinical end-points (BVS 4.9% vs. EES 7.0%, P=0.4); or individual endpoints including death (BVS 0.8%, EES 2.0%, P=0.4), MI (BVS 4.1%, EES 2.0%, P=0.2), target lesion revascularization (BVS 4.1%, EES 4.5%, P=0.8) or device thrombosis (BVS 2.5%, EES 1.4%, P=0.4). In addition, after careful propensity score matching, no differences in clinical endpoints were detected between BVS and EES at the longest available follow-up.
In an elegant study Brugaletta et al. (21) compared the results of BVS with those obtained with EES and BMS in the EXAMINATION trial. In this study the results of 290 consecutive STEMI patients treated with BVS were compared with those obtained in 290 STEMI patients treated with EES and 290 STEMI patients treated with BMS (21). A propensity score was used to adjust for potential confounders and obtain equally-sized groups of well-matched patients. Notably, pre and post-dilation was more frequently used in the BVS group. The primary end-point of the study was a device-oriented clinical outcome measure that included cardiac death, target vessel MI and target lesion revascularization. Interestingly, the primary end-point at 30 days and 1-year follow-up was low and similar (4.1%, 4.1% and 5.9%) for BVS, DES and BMS. Although, the rate of definitive/probable stent thrombosis was numerically higher for BVS than for DES or BMS, the differences were not statistically significant. However, the trend for a higher rate of early thrombosis after BVS compared with EES (2.1% vs. 0.3%, P=0.059) was a cause of concern (21).
On the other hand, results from head-to-head randomized comparisons of BVS vs. DES in patients with acute coronary syndrome are scarce. The EVERBIO II trial (22) randomized unselected “all-comers” patients, many of them presenting with an acute coronary syndrome (39% of patients but only 10% with STEMI), to BVS, EES or biolimus-DES. The primary endpoint of the study, the angiographic late lumen loss at 9-month follow-up, did not differ among the groups (0.28 mm in the BVS group, 0.25 mm in the DES groups). In addition, the combined clinical outcome measure was similar in the 3 arms. The ABSORB-STEMI-TROFI II was a multicenter randomized clinical trial that allocated (1:1) STEMI patients to BVS (95 patients) or EES (96 patients) (23). Randomization was performed after achievement of TIMI 2 flow following thrombus aspiration. In this trial thrombectomy was mandatory to reduce thrombus burden. Interestingly, postdilation was more frequently used in the BVS arm. Optical coherence tomography was used to compare arterial healing responses (non-inferiority design) with both devices as a surrogate for safety and efficacy. The primary endpoint was the comparison of the healing score (presence of uncovered struts, malapposed struts and intraluminal material) at 6 months assessed by optical coherence tomography (23). The healing score was lower (1.74 vs. 2.80, P for non-inferiority <0.001, P for superiority 0.053) in the BVS arm. This was mainly driven by a higher rate of uncovered and malapposed struts in the EES arm. However, the mean neointimal hyperplasia area was larger (1.52 vs. 1.35 mm2, P=0.018) in the BVS group. In addition, on quantitative coronary angiography, the mean in-device late lumen loss at 6 months was higher (0.17 vs. 0.08 mm, P=0.024) in the BVS arm. Importantly, a device-oriented composite end-point (cardiac death, target vessel MI and clinically-driven target lesion revascularization) (1.1% vs. 0%) and stent thrombosis rates (1.1% vs. 0%) were similar in the BVS and EES arms, respectively. This study demonstrated that BVS implantation in STEMI patients is associated with a nearly complete arterial healing at follow-up, with morphological findings comparable with those seen with EES (23). This is of potential clinical relevance considering previous studies suggesting superior healing characteristics of EES compared with first-generation DES.
The currently ongoing ISAR-ABSORB-MI randomized trial (NCT 194207) is comparing the safety and efficacy of BVS with durable polymer EES (2:1 randomization scheme) in patients with acute MI. Patients with STEMI and those with non-ST segment elevation MI associated with a clear angiographic thrombus, are eligible. The primary outcome measure is the comparison of the percentage diameter stenosis at the protocol-mandated coronary angiography performed at 6-8 month follow-up using a non-inferiority study design. Main secondary clinical endpoints include a device-oriented composite clinical endpoint of cardiac death, target vessel-MI and target lesion revascularization and a patient-oriented composite clinical endpoint of death, any MI and any revascularization. Enrolling a total of 260 patients is planned.
Conclusions
BVS are very appealing for selected STEMI patients. Results from available observational studies and randomized clinical trials are reassuring and very promising (16-23). Rates of early BVS thrombosis in adverse anatomic scenarios, including the presence of a highly thrombogenic milieu, however, appear to be not negligible. Therefore, careful lesion preparation, accurate scaffold sizing and, when required, postdilation, appear mandatory to ensure optimal BVS implantation in this complex scenario. Furthermore, thromboaspiration may be of particular value in patients with a large thrombus burden. In addition, the use of novel potent antiplatelet drugs is also highly appealing in these patients with enhanced platelet activity. The improvement in radial strength and reduction in strut thickness of new-generation BVS will hopefully represent a major step forward favouring optimal BVS deployment in this challenging anatomic setting. Current clinical data come from observational retrospective studies, registries with a control group of patients treated with DES, and randomized studies designed for surrogate primary end-points but not powered for major clinical events. As the potential advantages of BVS over metallic DES theoretically should accrue over time, a longer follow-up of the available studies will shed additional light on this issue. Meanwhile, the currently available information should be just considered as very promising but just hypothesis-generating. Further studies powered for clinical events are certainly needed to definitively establish the relative safety and efficacy of BVS versus new-generation metallic DES in STEMI patients.
Acknowledgements
None.
Footnote
Provenance: This is an invited article commissioned by the Section Editor Yue Liu (Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Rivero F, Bastante T, Cuesta J, et al. Factors associated with delays in seeking medical attention in patients with st-segment elevation acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2016;69:279-85. [Crossref] [PubMed]
- Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016;387:127-35. [Crossref] [PubMed]
- Alfonso F, Dutary J, Paulo M, et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart 2012;98:1213-20. [Crossref] [PubMed]
- Alfonso F, Fernandez C. Second-generation drug-eluting stents. Moving the field forward. J Am Coll Cardiol 2011;58:26-9. [Crossref] [PubMed]
- Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015;65:2496-507. [Crossref] [PubMed]
- Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380:1482-90. [Crossref] [PubMed]
- Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 2016;387:357-66. [Crossref] [PubMed]
- Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 2009;373:897-910. [Crossref] [PubMed]
- Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol 2014;64:2541-51. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43-54. [Crossref] [PubMed]
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144-53. [Crossref] [PubMed]
- Simsek C, Karanasos A, Magro M, et al. Long-term invasive follow-up of the everolimus-eluting bioresorbable vascular scaffold: five-year results of multiple invasive imaging modalities. EuroIntervention 2016;11:996-1003. [PubMed]
- Campos CM, Garcia-Garcia HM, Muramatsu T, et al. Impact of the everolimus-eluting bioresorbable scaffold in coronary atherosclerosis. Rev Esp Cardiol (Engl Ed) 2016;69:109-16. [Crossref] [PubMed]
- Brugaletta S, Radu MD, Garcia-Garcia HM, et al. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis 2012;221:106-12. [Crossref] [PubMed]
- Kajiya T, Liang M, Sharma RK, et al. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention 2013;9:501-4. [Crossref] [PubMed]
- Diletti R, Karanasos A, Muramatsu T, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J 2014;35:777-86. [Crossref] [PubMed]
- Cortese B, Ielasi A, Romagnoli E, et al. Clinical Comparison With Short-Term Follow-Up of Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Stent in Primary Percutaneous Coronary Interventions. Am J Cardiol 2015;116:705-10. [Crossref] [PubMed]
- Kočka V, Malý M, Toušek P, et al. Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: a prospective multicentre study 'Prague 19'. Eur Heart J 2014;35:787-94. [Crossref] [PubMed]
- Widimsky P, Petr R, Tousek P, et al. One-year clinical and computed tomography angiographic outcomes after bioresorbable vascular scaffold implantation during primary percutaneous coronary intervention for st-segment-elevation myocardial infarction: the PRAGUE-19 study. Circ Cardiovasc Interv 2015;8:e002933. [PubMed]
- Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv 2015;8:189-97. [Crossref] [PubMed]
- Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791-801. [Crossref] [PubMed]
- Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37:229-40. [Crossref] [PubMed]


