Ensartinib in advanced ALK-positive non-small cell lung cancer: a multicenter, open-label, two-staged, phase 1 trial
Highlight box
Key findings
• We report the results of safety, tolerability, pharmacokinetic (PK), efficacy and possible pharmacodynamics (PD) biomarkers of ensartinib of a phase I dose escalation and expansion trial in Chinese advanced ALK or ROS1 positive non-small-cell lung cancer (NSCLC) patients.
What is known and what is new?
• Ensartinib showed efficacy for patients with disease progression on crizotinib and superior efficacy to crizotinib in both systemic and intracranial disease.
• The recommended phase II dose (RP2D) decision, systemic PK and safety in dose escalation, and PD biomarkers of ensartinib in Chinese patients were firstly revealed.
What is the implication, and what should change now?
• We showed systemic PK, safety of different doses, pharmacodynamics and efficacy of ensartinib, which together with the results of Phase 2 and Phase 3 trials promoted the approval of ensartinib in ALK positive NSCLC patient treatment in China.
Introduction
Rearrangement of anaplastic lymphoma kinase (ALK) is observed in 3–11.6% of non-squamous non-small cell lung cancer (NSCLC) patients. ALK-positive NSCLC is associated with a more aggressive phenotype, a shorter time to recurrence, a poor prognosis (1-3) and are usually younger age with light or non-smoking history (4). About 30% of these patients were found with central nervous system (CNS) metastasis when first diagnosed (5). ALK inhibitors have shown efficacy and safety superiority than chemotherapy in ALK-positive NSCLC (6). Crizotinib is the first-generation ALK-tyrosine kinase inhibitor (TKI) (7,8). Despite the its initial efficacy, patients will eventually failed on crizotinib after about 12 months, with brain being the most frequent progression site and mostly disease progressed due to acquired secondary ALK mutations, such as L1196M, G1269A, C1156Y, L1152R, G1202R, S1206Y, 1151Tins, F1174C, and D1203N (9,10). In order to overcome acquired resistance and manage CNS localizations, several second- (ceritinib, alectinib and brigatinib) and third-generation (lorlatinib) TKIs have been developed. These ALK-TKIs have different potencies in inhibiting secondary ALK mutations, and can potentially overcome resistance (11).
Ensartinib (X-396) is a novel second-generation, aminopyridazine-based ALK-TKI which improve the activity on CNS metastases. Ensartinib can inhibit wild-type and ALK variants (F1174, C1156Y, L1196M, S1206R, T1151 and G1202R) as well as TPM3-TRKA, TRKC, ROS1, EphA2, EphA1, EphB1 and c-MET (12). In a phase I/II study, the recommended phase II dose (RP2D) of ensartinib was established to be 225 mg QD, with common drug-related adverse events including rash (56%), nausea (36%), pruritus (28%), and vomiting (26%); the objective response rate (ORR) was 60% and the median progression-free survival (mPFS) was 9.2 months (13). However, there was no Chinese patients enrolled in this trial, considering the ethnic differences, further studies are warranted. Therefore, phase I/II/III trials to assess the safety, efficacy, pharmacokinetic (PK) and possible biomarkers of ensartinib in Chinese advanced ALK-positive NSCLC patients were launched. In phase II and III study (14,15), ensartinib showed antitumor activities in patients failed on crizotinib, and superiority against crizotinib in systemic and intracranial efficacy, respectively.
Here, we report the results of safety, tolerability, PK, efficacy and possible PD biomarkers in the phase I dose escalation and expansion trial of ensartinib in Chinese advanced ALK or ROS1 positive NSCLC patients. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1606/rc).
Methods
Patients and study design
Patients were eligible for enrollment if they were between 18 and 70 years of age, and a pathological confirmed NSCLC, harbored ALK fusion or ROS1 mutation (confirmed by immunochemistry, FISH or gene sequence), had a performance status of 0 to 1 on the Eastern Cooperative Oncology Group (ECOG) scale, and had adequate bone marrow and organ function. The primary objectives of this trial were safety, RP2D determination of ensartinib based on tolerability and PK, secondary objectives including PK, efficacy and possible PD biomarkers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The two-center, open-label, two-staged, phase I trial was approved by the ethic committee of Sun Yat-Sen University Cancer Center (No. A2016-048-01) and the ethic committee of Zhejiang University School of Medicine Second Affiliated Hospital (No. 2017-018-IH). Informed consent was taken from all individual participants. This study was registered through ClinicalTrials.gov (NCT02959619).
Procedures
This study consisted of dose escalation and dose expansion phases. Dose escalation was based on a PK-guided 3+3 design. The starting dose was 150 mg, which was based on previous study (13). Patients orally received ensartinib administration at 150, 200, 225 and 250 mg dose levels once daily in 28-day cycles. Initially, each dose escalation cohort recruited 3–12 patients (determined by investigator and sponsor meeting based on safety or PK consideration). Dose limited toxicity (DLT) was assessed during the first 28-day cycle to determine maximum tolerated dose (MTD). After determination of the MTD and recommended dose, additional patients were enrolled in dose expansion stage and receive ensartinib at RP2D in 28-day cycles until disease progression or unacceptable toxicity. A DLT was defined as any of the following drug-related adverse events that occurred during the first treatment cycle: grade 4 neutropenia last >5 days or febrile neutropenia, grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding, grade 3–5 nonhematologic toxicity with the exception of grade 3 rash, diarrhea, nausea, or vomiting if controlled and resolved within 48 hours, or a treatment delay of >14 days due to unresolved toxicity. The MTD was defined as the highest dose level at which less than 1/3 patients experienced a DLT. The recommended dose of ensartinib would be 250 mg daily if no more than 1 DLT occurred, otherwise the MTD would be the recommended dose.
Assessment
Safety and efficacy
The safety and tolerability of ensartinib were assessed by evaluating vital signs, physical examination findings, performance status score, clinical laboratory testing (serum chemistry, hematology test, urinalysis, etc.), auxiliary examinations electrocardiogram (ECGs), visual history. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
At baseline, all patients underwent tumor imaging with CT and, if appropriate, magnetic resonance imaging (MRI). Brain imaging was required for patients with known or suspected brain metastases. Response evaluation was obtained at 8-week intervals, and assessed according to RECIST v1.1 by the investigators.
PK analyses
Patients were administered with a single dose of ensartinib in cycle 0 and received 7-day wash-out period, then administered continuously for multiple doses in cycle 1. PK analysis was performed in cycle 0 for single dose analysis and cycle 1 for multiple dose analysis. Systemic plasma samples were obtained from all patients in dose escalation and a subset of patients in the dose expansion phase before dosing of ensartinib (−0.5 h), and at 0.5, 1, 2, 3, 4, 8, 12, 16, 24, 36, 48, 72, 96, 120 and 144 h after dosing for single dose. On day 28 of cycle 1, systemic plasma samples were collected for multiple doses at same time points. Plasma samples were also collected prior (−0.5 h) to dosing on days 8, 15, and 22 of cycle 1. The concentration of ensartinib in plasma was determined by using a liquid chromatography-mass spectrometry (LC-MS) method (API 5500, Applied Biosystems, Waltham, MA, USA). PK parameters in plasma were calculated by non-compartmental analysis.
Biomarker analysis
Formalin-fixed paraffin-embedded (FFPE) tissues was collected voluntary at baseline, DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Peripheral blood samples were collected at baseline and C1D31 respectively, plasma and white blood cells DNA were extracted using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany) and the Hipure Blood&Tissue DNA Kit (Magen Biotechnology, Guangzhou, China). The DNA obtained was captured by SureSelect XT-HS Target Enrichment System (Agilent Technologies, Santa Clara, CA, USA) using a 212-gene panel (Table S1, Repugene Technology, Hangzhou, China) for all samples. The constructed libraries were sequenced with mean sequencing depths approximately 20,000 times for blood samples and 4,000 times for tissue samples using the Illumina HiSeq-X10 platform (Illumina, San Diego, CA, USA). Then, the samples and white blood cells were paired to filter out clonal hematopoiesis variants and germline mutations. MuTect2 was used for single-nucleotide variation and insert-deletion variant detection and LUMPY (version 0.2.13) was used for gene fusion detection (16). The copy number variation was detected using the CNVkit software (v0.9.5). Mutation load of each patient was the sum of the number of detected mutation and ALK mutation abundance was defined as the variant allele fractions of ALK mutations.
Statistical analysis
All patients who signed informed consent were included in full analysis set (FAS), safety analysis set (SS) included patients received at least one dose of ensartinib and has at least one post-baseline safety evaluation. Efficacy analyses were conducted in efficacy analysis set (10), which included patients who received at least one dose the study drugs and has at least one post-baseline tumor assessment. Toxicities were described by frequency and grade with the maximum grade over all cycles used as the summary measure per patient. Progression-free survival (PFS) was estimated using the Kaplan-Meier method and compared using the log-rank test. Mutation load between patients with TP53 mutations and patients without TP53 mutations was compared using Mann-Whitney U test. Genetic factors related to PFS were determined using Univariate Cox regression analyses. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses was performed on genes detected with nonsynonymous mutations in long-PFS and short-PFS group using the R cluster Profiler package. Enriched pathways with P values <0.05 were considered significant. The analyses were conducted with SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Patients characteristics
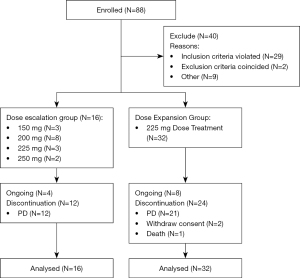
As showed in Figure 1, forty-eight patients (16 in dose escalation and 32 in dose expansion) were enrolled from March 6, 2017 to October 31, 2019. Baseline demographics are presented in Table 1. The median age was 48 (range, 23–77). Of 48 enrolled patients, 43 patients were detected with ALK rearrangement and 5 patients harbored with ROS1 fusion. Eleven (22.9%) patients were prior ALK TKI treated, among them 2 (4.2%) received second-generation ALK inhibitor treatment. Sixteen (33.3%) patients had brain metastases at baseline (Table 1).
Table 1
| Characteristics | Ensartinib dose cohorts (mg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 (N=3) | 200 (N=8) | 225 (N=35) | 250 (N=2) | Total (N=48) | ||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||||
| Median age, years (range) | 52.3 (40–64) | 49 (37–77) | 46.8 (23–65) | 44 (41–49) | 47.5 (23–77) | |||||||||
| Gender | ||||||||||||||
| Female | 1 | 33.3 | 3 | 37.5 | 20 | 57.1 | 1 | 50 | 25 | 52.1 | ||||
| Male | 2 | 66.7 | 5 | 62.5 | 15 | 42.9 | 1 | 50 | 23 | 47.9 | ||||
| ECOG performance status | ||||||||||||||
| 0 | 1 | 33.3 | 3 | 37.5 | 18 | 51.4 | 2 | 100 | 21 | 43.8 | ||||
| 1 | 2 | 66.7 | 5 | 62.5 | 17 | 48.6 | 0 | 0 | 27 | 56.2 | ||||
| Smoking status | ||||||||||||||
| Yes | 1 | 33.3 | 2 | 25 | 9 | 25.8 | 1 | 50 | 12 | 25 | ||||
| No | 2 | 66.7 | 6 | 75 | 26 | 74.2 | 1 | 50 | 36 | 75 | ||||
| ALK positive | 3 | 8 | 30 | 2 | 43 | |||||||||
| ROS1 positive | 5 | 5 | ||||||||||||
| Ensartinib as first-line treatment | 1 | 33.3 | 6 | 75 | 23 | 17.1 | 2 | 100 | 15 | 31.3 | ||||
| No prior ALK-TKIs treatment | 1 | 33.3 | 6 | 75 | 28 | 17.1 | 2 | 100 | 37 | 77.1 | ||||
| Prior ALK-TKIs treatment | 2 | 66.7 | 2 | 25 | 7 | 82.9 | 0 | 0 | 11 | 22.9 | ||||
| Median No. of metastatic organs (range) | 2 (1–3) | 3 (1–5) | 3 (1–6) | 3 (1–5) | 3 (1–6) | |||||||||
| No. of prior metastatic anticancer regimens | ||||||||||||||
| <3 | 3 | 100 | 8 | 100 | 32 | 91.4 | 2 | 100 | 45 | 93.7 | ||||
| ≥3 | 0 | 0 | 0 | 0 | 3 | 8.6 | 0 | 0 | 3 | 6.3 | ||||
| No. of patients with brain metastases | 1 | 33.3 | 4 | 50 | 11 | 31.4 | 1 | 50 | 17 | 35.4 | ||||
ECOG, Eastern Cooperative Oncology Group; ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor.
Safety, tolerability and RP2D
As showed in Table 2, among all the treatment-related adverse events (TRAEs), 27% (13/48) were grade 3–5. The most common TRAEs were rash (87.5%), increased alanine aminotransferase (ALT) (60.4%), increased aspartate transaminase (AST) (54.2%), pruritus (45.8%), increased serum creatinine (35.4%), leukocytosis (29.2%), skin exfoliation (27.1%), vomiting (27.1%), dermatitis (25.0%), nausea (25.0%), neutrocytosis (20.8%). The most frequent grade 3–5 TRAEs were rash (14.6%) and increased ALT (12.5%). Most TRAEs were grade 1 to 2. Dose reduction and delay of ensartinib was seen in 9 and 13 patients, respectively. Serious AEs (SAEs) occurred in 10 (20.8%) patients. Rash (2 patients; 4.2%), facial edema (1 patient; 2.1%) and hepatic failure (1 patient; 2.1%) were considered treatment related. Two DLTs were observed in the 250 mg dose cohort (grade 3 rash) and the RP2D of ensartinib in Chinese NSCLC patients was determined to be 225 mg.
Table 2
| TRAE | Ensartinib dose cohorts (mg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 (N=3) | 200 (N=8) | 225 (N=35) | 250 (N=2) | Total (N=48) | ||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||||
| ≥3 grade TRAE | 0 | 0 | 2 | 25 | 9 | 25.7 | 2 | 100 | 13 | 27.1 | ||||
| Rash | 2 | 66.7 | 6 | 75 | 32 | 91.4 | 2 | 100 | 43 | 87.5 | ||||
| ≥3 grade rash | 0 | 0 | 0 | 0 | 5 | 14.3 | 2 | 100 | 7 | 14.6 | ||||
| Increased ALT | 1 | 33.3 | 5 | 62.5 | 22 | 62.9 | 1 | 50 | 29 | 60.4 | ||||
| ≥3 grade increased ALT | 0 | 0 | 2 | 25 | 4 | 11.4 | 0 | 0 | 6 | 12.5 | ||||
| Increased AST | 1 | 33.3 | 5 | 62.5 | 19 | 54.3 | 1 | 50 | 26 | 54.2 | ||||
| ≥3 grade increased AST | 0 | 0 | 1 | 12.5 | 1 | 2.9 | 0 | 0 | 2 | 4.2 | ||||
| Pruritus | 1 | 33.3 | 4 | 50 | 17 | 48.6 | 0 | 0 | 22 | 45.8 | ||||
| Leucocytosis | 0 | 0 | 4 | 50 | 10 | 28.6 | 0 | 0 | 14 | 29.2 | ||||
| Neutrocytosis | 0 | 0 | 2 | 25 | 8 | 22.9 | 0 | 0 | 10 | 20.8 | ||||
| Increased creatinine | 0 | 0 | 4 | 50 | 12 | 34.3 | 1 | 50 | 17 | 35.4 | ||||
| Skin exfoliation | 0 | 0 | 3 | 37.5 | 10 | 28.6 | 0 | 0 | 13 | 27.1 | ||||
| Dermatitis | 0 | 0 | 3 | 37.5 | 8 | 22.9 | 1 | 50 | 12 | 25.0 | ||||
| Vomiting | 0 | 0 | 0 | 0 | 12 | 34.3 | 1 | 50 | 13 | 27.1 | ||||
| Nausea | 0 | 0 | 2 | 25 | 9 | 25.7 | 1 | 50 | 12 | 25.0 | ||||
| Constipation | 0 | 0 | 0 | 0 | 3 | 8.6 | 0 | 0 | 3 | 6.3 | ||||
| Diarrhea | 0 | 0 | 0 | 0 | 7 | 20 | 0 | 0 | 7 | 14.6 | ||||
| Fever | 0 | 0 | 0 | 0 | 5 | 14.3 | 0 | 0 | 5 | 10.4 | ||||
| Dizziness | 1 | 33.3 | 0 | 0 | 2 | 5.7 | 1 | 0 | 3 | 6.3 | ||||
| Albuminuria | 0 | 0 | 0 | 0 | 5 | 14.3 | 0 | 0 | 5 | 10.4 | ||||
| Oral ulcer | 1 | 33.3 | 2 | 25 | 5 | 14.3 | 1 | 50 | 9 | 18.8 | ||||
| Abdominal pain | 0 | 0 | 0 | 0 | 5 | 14.3 | 0 | 0 | 5 | 12.5 | ||||
Ensartinib-related adverse events include definitely related AEs and probably related AEs. TRAE, treatment related adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AEs, adverse events.
PK analyses
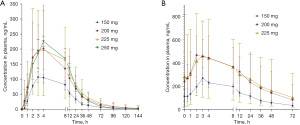
PK analysis was performed in cycle 0 for single dose analysis and cycle 1 for multiple dose analysis (Table S2). In single dose administration (150–250 mg), the area under the curve (AUC) reached the highest at 200 to 225 mg dose. Compared to the AUC of 225 mg, the AUC of 150, 200, 250 mg was 0.51, 0.96, 1.11 folds (R2 of dose versus exposure linear regression graph for was 0.7707) at the steady state. The absorption of ensartinib was relatively slow with a median Tmax of 3.00 to 4.00 hours; mean T1/2 ranged from 21.0 to 30.2 hours; mean Cmax ranged from 110 to 225 ng/mL. In multiple dose administration (150–225 mg), a steady-state concentration of ensartinib was reached after 8–15 days. The mean residence time (MRT) [± standard deviation (SD)] of 150, 200, 225 mg dose cohort was 2.76±0.626, 3.08±1.60, 3.43±2.03 h × ng/mL. Median time to reach maximum plasma concentration (Tmax) was 2–3 h, with mean Cmax ranging from 265 to 435 ng/mL. The T1/2 ranged from 28.4 to 35.4 h. In single and multiple dose administration, the AUC0-t, AUC0-tau, Cmax, Ctrough tend to increase with ascending dose, but the accumulation pattern of ensartinib was not completely linear (Figure 2). No clear correlation was noted between ensartinib exposure and age, sex, ethnic organ, and body-mass index within every dose cohort (data not shown).
Anti-tumor activity
A summary of the confirmed best overall response on the basis of investigator review is provided in Table S3 and Figure 3. In all patients (43 ALK+ and 5 ROS1+), the ORR and disease control rates (DCR) were 64.6 % (1 CR and 30 PR) and 81.3% (8 SD), respectively. The median PFS was 16.79 months [95% confidence interval (CI), 8.11 to 25.47 months]. For patients treated with ensartinib ≥225 mg (37 patients), the ORR and DCR were 62.2% (95% CI, 45.8–78.6%) and 78.4% (95% CI, 64.5–92.3%), while ORR and DCR for patients with ensartinib <225 mg (11 patients) were 72.7% (95% CI, 41.3–100%) and 90.9% (95% CI, 70.7–111%). The median PFS in 150 and 200 mg cohorts was 2.07 months (ranged, 1.15 to 16.79 months) and 26.88 months (95% CI, 2.29–51.46 months), while in 225 mg and 250 mg cohorts the mPFS was 16.62 months (95% CI, 6.11–26.14 months) and 22.82 months (ranged,18.23 to 38.37 months).
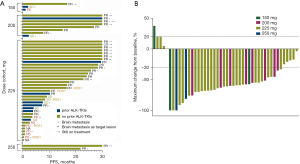
In 43 ALK positive patients, the ORR and DCR were 72.1% (95% CI, 58.1–86.1%) and 83.7% (95% CI, 72.2–95.2%), respectively. The mPFS was 18.23 months (95% CI, 8.77–27.70 months) (Table S4). In ALK TKI-naïve patients, the confirmed ORR was 81.3% (26/32) compared to 45.5% (5/11) in ALK TKI-resistant patients, mPFS was 25.73 months (95% CI, 20.41–31.04 months) and 4.14 months (95% CI, 0.00–8.75 months) respectively. While in 5 ROS1 positive patients, the ORR and DCR were 0% and 60% (3 SD). Ensartinib showed disease control in patients with brain metastases (Figure 3). The ORR and DCR in 16 patients with baseline brain metastases were 68.8% (95% CI, 43.2–94.3%) and 68.8% (95% CI, 43.2–94.3%) respectively, median PFS was 7.59 months (95% CI, 6.75–8.43 months). In 3 patients with measurable brain metastasis lesions, the ORR were 66.7% (2 PR and 1PD), median intra-cranial PFS was 22.90 months (ranged, 3.65 to 30.36 months) .
Biomarker analyses
Baseline mutation landscape
All genes detected with nonsynonymous mutations at baseline were displayed in Figure 4. TP53 and KMT2C had the highest mutation rates, both detected in 5 patients (5/27, 19%). After one cycle of treatment, the number of mutant genes detected at C1D31 was greatly reduced (Figure S1). Notably, the frequency of ALK mutations remarkably decreased from 100% at baseline to 5% at C1D31.
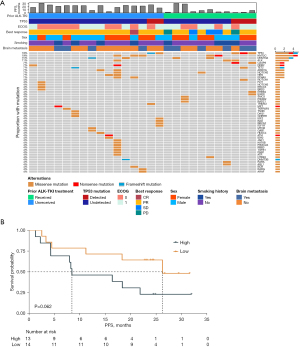
The median ALK mutation abundance at baseline was taken as cutoff (median =0.000573148) to define high or low abundance. The median PFS was 26.25 months (95% CI, 4.50 to not estimable) and 8.46 months (95% CI, 4.25–21.9 months) in patients with low and high ALK mutation abundance (Figure 4). Although there was marginally statistical significance between two groups [hazard ratio (HR), 0.39; 95% CI, 0.14–1.09; log-rank test P=0.06], ALK abundance at baseline showed high predictive value of ensartinib anti-tumor activity.
Change of mutation landscape during the course of treatment
Among 43 patients with advanced disease, 21 patients (48.84%) underwent continuous ctDNA testing at baseline and C1D31. Change in the mutation profile was evident during the treatment (Figure S1). At baseline, nonsynonymous mutations were detected in 50 genes. At C1D31, only 2 baseline mutations remained, 21 de novo mutations occurred. At baseline, patients with TP53 mutation had higher gene mutation load (TP53 mutant group, 4.25±0.96 versus TP53 wild-type group, 1.76±1.44, P=0.010). At C1D31, the mutation load of the two group has not significant difference (TP53 mutations detected group, 1.25±1.26 versus TP53 mutations undetected group, 1.06±1.09, P=0.779).
KEGG analysis
Taking 9 months as the cut-off time, the patients were divided into long PFS group (PFS >9 m) and short PFS group (PFS >9 m). KEGG analysis was performed on the two groups of all genes detected with nonsynonymous mutations (Figure S2). Pathways that were only enriched in the long PFS group were screened as signaling pathways (MAPK, FoxO, HIF-1 and Rap-1), focal adhesion and virus carcinogenesis; and pathways that only enriched in the short PFS group were screened as p53 signaling pathway, apoptosis, notch signaling pathway and chemical carcinogenesis-receptor activation (Figure S3).
Discussion
In this phase I study, we found that ensartinib ≤225 mg once per day was safe and well tolerated in Chinese NSCLC patients. Results in dose escalation supported 225 mg once per day as the RP2D. Also, systemic PK results indicated that the AUC of ensartinib reached saturation at 225 mg. Safety analysis indicated that common TRAEs was rash, transaminase elevation and pruritus, which was consistent with phase II/III trials. Once again, ensartinib proved its anti-tumor activity in ALK+ NSCLC patients in TKI-naïve or resistant patients. The biomarkers analysis suggested patients with higher ALK mutation abundance at baseline were likely benefit less in ensartinib treatment.
The main TRAEs were grade 1 or 2 rash, which was consistent with reports of ensartinib from phase I/II/III multicenter studies (13-15). TRAEs could be successfully managed by dose suspension or reduction. Most patients did not require dose adjustment during the treatment. Ensartinib appears to have a different safety profile from other ALK-TKIs. Previous studies showed that gastrointestinal toxicities (nausea, diarrhea, and vomiting) were common in patients after crizotinib treatment. While diarrhea was reported low incidence in patients treated with ensartinib, which was significantly lower than those in patients who received brigatinib (17) or ceritinib (18). Moreover, the frequency and severity of elevated aminotransferases reported as TRAEs with ensartinib was also lower than those with other ALK inhibitors (17-20). The incidence of grade 3 or higher TRAEs reported in this study was consistent with crizotinib (21) and less than ceritinib (22) and brigatinib (18). Overall, the safety of ensartinib was acceptable.
The PK analysis demonstrated that the plasma exposure of ensartinib may not increase proportionally as dose escalated. Notably, the half-life of ensartinib in the present study was consistent with previous reported results of alectinib, brigatinib and lorlatinib but was shorter compared to crizotinib or ceritinib, respectively (23-26). A short half-life allows ensartinib quickly decrease in the plasma thus reducing the drug or active metabolites accumulation, which might improve safety and decrease TRAEs of ensartinib.
Ensartinib once again demonstrated satisfy efficacy in patients with ALK positive NSCLC patients. In this study, the included patients were prior treated with one or more lines of chemotherapy, or with ALK-TKIs, yet the ORR and clinical benefit rate (CBR) were impressive. The median PFS and ORR of ensartinib in ALK TKI-naïve patients were consistent with alectinib and might higher than ceritinib (20,22). A high risk of developing brain metastases was found in patients with ALK-positive NSCLC. About 30% of cases were found brain metastasis when first diagnosed (27). It’s worth mentioning that, in this study, the ORR and DCR in 16 patients with baseline brain metastases were comparable with those of ceritinib, alectinib and brigatinib (9,23,28,29).
The gene mutation landscape showed that the most common co-mutation genes of ALK positive patients in our study were TP53 and KMT2C, which were reported functions in cell proliferation, tumor formation and DNA damage repair (30-34). At baseline, patients with TP53 mutation had higher gene mutation load, which was reported associated with primary resistance to crizotinib in ALK+ NSCLC (35). We found that patients with low ALK mutation abundance at baseline achieved significantly longer mPFS compared to those with high ALK mutation abundance. KEGG analysis showed that mutations enriched only in the short PFS group, which may results in therapeutic resistance and cancer progression through chronic inflammation (36,37). Due to limited sample size, the relationship among TP53 mutation, mutation load, ALK mutation abundance with mPFS in ensartinib treatment should be clarified in perspective studies with more subjects.
Conclusions
In conclusion, ensartinib demonstrated good clinical activity and an acceptable safety profile in Chinese ALK-positive NSCLC patients with or without prior ALK TKIs treatment, and with CNS metastases.
Acknowledgments
We thank the participating patients and their families and contributing clinical staff across all sites. This study is supported by Betta Pharmaceuticals Co., Ltd. Labcorp Pharmaceutical Research & Development (Shanghai) Co., Ltd., Hangzhou Repugene Technology Co., Ltd., XenoBiotic Laboratories,Inc (Nanjing, China) provided bioanalytical assessments. Medical writing services were provided by Meta Clinical Technology Co. Ltd. (Xian, China) and were funded by Betta Pharmaceuticals Co., Ltd. Jianjin Huang and Shanshan Weng, Professors of the Department of Oncology at The Second Affiliated Hospital Zhejiang University School of Medicine also assisted in the writing of this manuscript. We would like to thank professor Christopher Lavender for his help in polishing our paper.
Funding: This study (clinical trial identification: NCT02959619) was sponsored by Betta Pharmaceuticals Co., Ltd., Hangzhou, China. This study was supported by National Nature Science Foundation of China (No. 82002409 to Yuxiang Ma, No. 82073396 to Hongyun Zhao, and No. 81872449 for Li Zhang), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2020A1515010020 to Yuxiang Ma, and Grant No. 2018A0303130243 to Hongyun Zhao).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1606/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1606/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1606/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1606/coif). SW, LZ, and HZ report funding from Betta Pharmaceuticals Co., Ltd. SW reports that the fund was managed by Zhejiang University School of Medicine Second Affiliated Hospital. LD and LC report that they are employees of Betta Pharmaceuticals Co., Ltd. SX and TW report that they are employees of Hangzhou Repugene Technology Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethic committee of Sun Yat-Sen University Cancer Center (No. A2016-048-01) and the ethic committee of Zhejiang University School of Medicine Second Affiliated Hospital (No. 2017-018-IH). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Shi Y, Sun Y. Medical management of lung cancer: Experience in China. Thorac Cancer 2015;6:10-6. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Khan M, Lin J, Liao G, et al. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front Oncol 2018;8:557. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Yanagitani N, Uchibori K, Koike S, et al. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci 2020;111:932-9. [Crossref] [PubMed]
- Li T, Ma W, Tian EC. Ensartinib (X-396): what does it add for patients with ALK-rearranged NSCLC. Chin Clin Oncol 2019;8:S4. [Crossref] [PubMed]
- Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin Cancer Res 2018;24:2771-9. [Crossref] [PubMed]
- Yang Y, Zhou J, Zhou J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med 2020;8:45-53. [Crossref] [PubMed]
- Horn L, Wang Z, Wu G, et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol 2021;7:1617-25. [Crossref] [PubMed]
- Layer RM, Chiang C, Quinlan AR, et al. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol 2014;15:R84. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer 2006;6:546-58. [Crossref] [PubMed]
- Hamilton G, Rath B, Burghuber O. Pharmacokinetics of crizotinib in NSCLC patients. Expert Opin Drug Metab Toxicol 2015;11:835-42. [Crossref] [PubMed]
- Morcos PN, Yu L, Bogman K, et al. Absorption, distribution, metabolism and excretion (ADME) of the ALK inhibitor alectinib: results from an absolute bioavailability and mass balance study in healthy subjects. Xenobiotica 2017;47:217-29. [Crossref] [PubMed]
- Zhao D, Chen J, Chu M, et al. Pharmacokinetic-Based Drug-Drug Interactions with Anaplastic Lymphoma Kinase Inhibitors: A Review. Drug Des Devel Ther 2020;14:1663-81. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [Crossref] [PubMed]
- Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol 2011;2011:583929. [Crossref] [PubMed]
- Ford DJ, Dingwall AK. The cancer COMPASS: navigating the functions of MLL complexes in cancer. Cancer Genet 2015;208:178-91. [Crossref] [PubMed]
- Lee JE, Wang C, Xu S, et al. H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation. Elife 2013;2:e01503. [Crossref] [PubMed]
- Lee J, Kim DH, Lee S, et al. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc Natl Acad Sci U S A 2009;106:8513-8. [Crossref] [PubMed]
- Xiao D, Deng Q, He D, et al. High Tumor Mutation Burden and DNA Repair Gene Mutations are Associated with Primary Resistance to Crizotinib in ALK-Rearranged Lung Cancer. Onco Targets Ther 2021;14:4809-17. [Crossref] [PubMed]
- Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021;6:263. [Crossref] [PubMed]
- Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 2020;119:199-245. [Crossref] [PubMed]