Inferior pulmonary ligament approach and/or interlobar fissure approach for posterior and/or lateral basal segment resection: a case-series of 31 patients
Highlight box
Key findings
• Optimizing the procedure of the inferior pulmonary ligament approach can reduce the surgical trauma and postoperative complication in posterior and/ or lateral basal segment resection.
What is known and what is new?
• The traditional interlobar fissure approach is easy to exposure but difficult to separate, especially the space between the dorsal segment and the basal segment.
• The inferior pulmonary ligament approach is relatively simple operation, easy to master, no need to separate the lung tissue between the dorsal segment and the basal segment, little trauma and short time, avoiding the risk of pulmonary torsion after operation, and high operator’s operation fluency and comfort.
What is the implication, and what should change now?
• In posterior and/ or lateral basal segment resection, we recommend the approach of mainly passing through the inferior pulmonary ligament, supplemented by the interlobar fissure approach when any difficulty in judgment occurred during the operation.
Introduction
With the popularization of low-dose spiral computed tomography (CT) (1,2), the detection rate of peripheral pulmonary nodules and multiple nodules, especially early lung cancer, is increasing every year. Anatomical lobectomy has the same therapeutic effect as traditional lobectomy (3-5) and is increasingly favored by doctors and patients, especially for patients in whom wedge resection of deep pulmonary nodules cannot be performed. For patients with multiple pulmonary nodules or poor lung function, segmentectomy is more appropriate. Simple segmentectomy is relatively easy, while complicated segmentectomy, such as posterior and/or lateral basal resection, presents a considerable challenge for many thoracic surgeons because of its high surgical difficulty and high incidence of postoperative complications.
The present study summarizes and analyzes the surgical skills and points of attention of thoracoscopic posterior and/or lateral basal segment resection via the inferior pulmonary ligament approach and/or interlobar fissure approach to further explore the characteristics of these two surgical methods and help operators choose and apply them. Here, we recommend thoracic surgeon choose the inferior pulmonary ligament approach for posterior and/or lateral basal resection because it optimizes the surgical process and reduces surgical trauma and postoperative complications. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1719/rc).
Methods
Data and methods
Clinical data
Included patient clinical data were retrospectively collected between January 2020 and June 2022. The inclusion criteria were: 18–75 years old; intersegmental or intrasegmental nodule in posterior and/or lateral basal segment, consolidation tumor ratio <0.5 and total diameter ≤2 cm. Routine preoperative examinations see below for details. Follow-up was continued to September 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Sichuan Provincial People’s Hospital (No. 2021-516). Individual consent for this retrospective analysis was waived.
Preoperative examination
Required: hematology, electrocardiogram, and chest thin-layer CT.
Optional: cardiac ultrasound, lung function, or head and upper abdomen CT or magnetic resonance imaging (MRI).
For elderly patients (≥70 years old) or patients with long-term hypertension and diabetes mellitus (>5 years), coronary CT angiography or coronary angiography was needed.
According to the results of the thin-layer chest CT examination, there were 20 single lesions and 11 multiple lesions among the 31 patients. Also, there were 2 cases of type 2 diabetes mellitus, 1 case of old pulmonary tuberculosis, 2 cases of hypertension, and 1 case of coronary heart disease before the operation (Table 1).
Table 1
| Clinical characteristics | Total (N=31) |
|---|---|
| Age (years) | |
| Range | 31–62 |
| Median | 51 |
| Male/female | 7/24 |
| Comorbidity | |
| Diabetes | 2 |
| Old tuberculosis | 1 |
| Hypertension | 2 |
| Coronary heart disease | 1 |
| Lesion | |
| Single | 20 |
| ≥2 | 11 |
Surgical indications
- Intrasegmental nodule and intersegmental nodule in posterior and/or lateral basal segment. Intersegmental nodule (6): the nodule is located at the edge of the lung segment or between adjacent lung segments. On the image, the minimum distance between this type of pulmonary nodule and the related intersegmental vein is less than or equal to the maximum diameter of the nodule. Therefore, anatomical single-segmental resection often cannot guarantee the safety of the edge of the lung segment or the cutting edge of the intersegmental nodule, so combined segmental resection or wedge resection of a single segment plus adjacent segments is often performed.
- Lesions ≤2 cm, and ground-glass opacity (GGO) components ≥50%.
- Regardless of the proportion of GGO components, it is considered a benign lesion before the operation and can be treated by segmental resection.
Preoperative planning
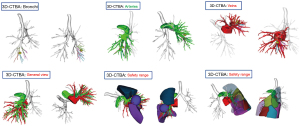
Using thin-slice CT examination of the chest and/or three-dimensional CT bronchography and angiography (3D-CTBA), the size, location, and imaging diagnosis of the lesion were clearly defined, and preoperative planning was completed (focusing on the lesion and based on the spatial relationship between bronchi, arteries, and veins of the lesion lung segment; tracing the root and source; accurately determining the ownership of the lesion lung segment and reliable resection margins; thus, determining the operation mode, resection range, and anatomical process of the segment portal structure, etc.) (Figures 1-3).


Anesthesia method
This process combined intravenous anesthesia, double-lumen endotracheal intubation, and unilateral controlled pulmonary ventilation. According to the intraoperative situation, the lungs may be interrupted or the breathing may be controlled manually, and the high frequency and low tidal volume ventilation strategy may be considered for breathing.
Surgical methods
Surgical incision
The improved two-hole method was mainly used (the main operation hole: the fourth or fifth intercostal space between anterior axillary and midline, approximately 4.0 cm long; the lens hole of the endoscope and the auxiliary operation hole were combined into one: the 7th or 8th intercostal space of the middle-posterior axillary line, approximately 2.0 cm long) (Figure 4). A partial three-hole method (improved two-hole method + auxiliary operation hole: anterior axillary-midline 6th or 7th intercostal space, approximately 2.0 cm long) was also used.
Body position: the positive side-lying position
Operation process
After entering the chest, the chest cavity was first explored and thoracic adhesions were dealt with. Based on experience, a three-dimensional anatomical localization method can be used to mark nodules on the lung surface.
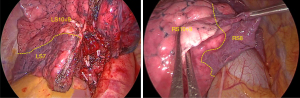
Inferior pulmonary ligament approach (7-9) (Figure 5): to optimize the procedure and avoid repeated flipping of the lung during surgery, the 7th group of lymph nodes was sampled first, and then the small gauze piece was clamped using sponge forceps to pull the lung to the head side. Next, the aspirator pressed the diaphragm top, the inferior pulmonary ligament was loosened, the 9th group of lymph nodes was taken, the branches of the inferior pulmonary vein were dissected, and the target veins (V10, V9) were identified and ligated and/or hemmed with a suture after separation. The target veins were cut off using an ultrasonic knife, and the intersegmental veins were preserved. Above the veins are the posterior and/or lateral basal segment bronchus (B10, B9); the target segment bronchi and 13th group of lymph nodes were taken, and the target segment bronchi were separated and treated with a stapler (choose staple cartridge with different thicknesses according to the different thicknesses of the bronchi, mostly blue or gold treatment). To adjust the bronchial angle and facilitate the placement of the stapler, the target bronchus can be bypassed (10) with a suture or guided using an aspirator or a vascular clamp. After cutting off the target bronchi, the target arteries (A10, A9) can be seen above them. The 12th group of lymph nodes can subsequently be taken. After cutting off the target arteries and ligating them with a suture or Hem-o-lock, the ultrasonic knife can be cut off, and the thicker arteries can also be treated with a stapler.
Interlobar fissure approach (11-13) (Figure 6): the inferior pulmonary trunk can be dissected from the interlobar fissure, and the dorsal artery (A6) and basilar arteries can be exposed and identified. After determining the A6, the dorsal bronchus (B6) is located below it, and the B10+9 bronchus lateral wall is located in the front and lower part of B6. After dissecting backward and downward, the dorsal vein (V6) can be exposed at the hilum, an intersegment tunnel can be established along the root of V6, and S6 and the basal segment are separated along this tunnel with a stapler. The door of the base section can then be exposed, and then the posterior and/or lateral basal arteries, bronchi, and veins can be separated downward.
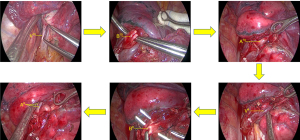
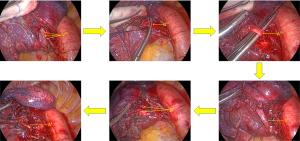
Exposure and treatment of the intersegmental plane: the intersegmental boundary can be defined via the improved expansion and collapse method (14). The process was performed as follows: (I) under the condition of one-lung ventilation on the healthy side, after accurately cutting off the target artery and bronchus, the anesthesiologist manually controlled pure oxygen ventilation in both lungs. The airway pressure was 20 cmH2O until the operation-side lung was completely inflated, and the healthy side one-lung ventilation was subsequently resumed; (II) after waiting for approximately 15 min, the boundary line between the target segment and adjacent lung segments on the pleura can be seen (the reserved lung segment is completely collapsed in dark red, and the target segment is partially expanded in pink) (Figure 7).
After exposing the intersegmental plane, the distal bronchus and artery end of the target segment can be pulled and lifted. Then, the interface of expansion and collapse from the segment gate to the distal end along the intersegmental vein can be dissected and separated, and electrocoagulation can be applied. An ultrasonic knife and scissors can then be utilized to sharply separate the interface between segments, cutting off the intrasegmental veins into the expanded lung tissue along the way. Since the three-dimensional structure of the lung segment is mostly small inside and large outside of the vertebral body, after processing the segment door and fully separating the lung parenchyma, the lung parenchyma at the interface between the segments on both sides can be opened from inside to outside using a stapler, so that the plane between segments can be stretched out for easy processing. Moreover, as the connective tissue between segments of the lung gradually decreases from the segment portal to the periphery, the alveolar communication is dense at 1/4–1/3 of the periphery. Therefore, when the boundary between lung segments is separated from the lung parenchyma (3-dimension is reduced to 2-dimension), the stapler can be used to cut in different directions and angles to minimize shrinkage of the remaining lung.
Following resection of the lung segment, warm sterilized water was injected into the chest cavity, and air leakage of the wound was observed by swelling of the lung. The airway pressure was 15–20 cmH2O; a small amount of air leakage wound was covered in soluble hemostatic gauze, and fibrin glue was sprayed. Serious air leakage should be carefully sewn and tied first, and if necessary, the wound should be covered.
According to intraoperative freezing, in cases of atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS), the corresponding hilar and mediastinal lymph nodes should not be sampled or cleaned. If the sample is identified as a microinvasive adenocarcinoma (MIA) by frozen section, the corresponding regional lymph nodes need to be sampled and sent for inspection [Groups 7, 9, 10, 12, and 13 (optional)]. However, if it is identified as an invasive adenocarcinoma (IA), it is necessary to communicate with family members and choose to perform lobectomy and lymph node dissection at the same time [left side: Groups 4 L, 5, 6, 7, 8, 9, 10, and 11; right side: Groups 2, 4R, 3A (optional), 7, 8, 9, 10, and 11] or only lymph node dissection.
Before closing the chest, an intercostal nerve block was routinely performed in the 3rd – 9th intercostal area; a thoracic drainage tube was placed in the endoscopic hole to the top of the chest, and the incision was closed layer by layer to complete the procedure.
Results
There were 31 patients in this study cohort, including 7 males and 24 females, with a median age of 51 [31–62] years. Among these patients, there were 25 cases of lung cancer and 6 cases of benign pulmonary nodules (Table 1).
All patients successfully completed the operation, without conversion to thoracotomy, and one patient was converted to lobectomy due to insufficient incision margins intraoperatively. In this cohort, two patients were treated using the interlobar fissure approach, 25 patients were treated via the inferior pulmonary ligament approach, and four patients were treated by employing both methods. Also, 20 patients had a single lesion, and 1 patient had two lesions. Eleven patients underwent surgical resection on other lesions when posterior and/or lateral basal segment lesions resected (Table 2).
Table 2
| Category | No. of cases |
|---|---|
| Surgical approaches | |
| Interlobar fissure (A) | 2 |
| Inferior pulmonary ligament (B) | 25 |
| A + B | 4 |
| Surgical types | |
| RS10 | 5 |
| RS10 + RULW | 2 |
| RS10 + LLLW | 1 |
| RS10 + RMLW | 1 |
| RS10 + LS1+2 + LLLW | 1 |
| RS10+9 | 3 |
| RS10+9→RLL + RS2 | 1 |
| RS10+9 + RULW | 1 |
| LS10 | 5 |
| LS10 + LULW | 1 |
| LS9 | 1 |
| LS9 + LULW | 1 |
| LS10+9 | 5 |
| LS10+9 + RS1 | 1 |
| LS10+6 | 1 |
| LS10+9+6 | 1 |
RULW, right upper lobe wedge resection; LLLW, left lower lobe wedge resection; RMLW, right middle lobe wedge resection; RLL, right lower lobe resection; RULW, right upper lobe wedge resection; LULW, left lower lobe wedge resection; RS1, apical segment of the right upper lobe resection.
The median operation time was 120 [50–290] min, the median intraoperative blood loss was 50 [10–100] mL, the median postoperative drainage time was 4 [2–10] days, and the median postoperative hospital stay was 4 [2–13] days. There were no perioperative deaths, and the postoperative complications included five cases of persistent air leakage longer than 5 days (7, 7, 8, 9, and 10 days). One patient developed a pulmonary infection and abnormal liver function and was discharged from the hospital on the 13th day after treatment. Postoperative pathology showed that there were 25 cases of primary adenocarcinoma or atypical adenomatous hyperplasia, including 13 cases of MIA, 11 cases of IA, and 1 case of AAH. The median maximum diameter of the lesion was 0.8 [0.2–1.5] cm, 8 [4–15] lymph nodes were resected, and no tumor metastasis was found. Inflammatory lesions were detected in six cases (Table 3).
Table 3
| Variables | Total (N=31) |
|---|---|
| Operative time (min) | |
| Range | 50–290 |
| Median | 120 |
| Blood loss (mL) | |
| Range | 10–100 |
| Median | 50 |
| Chest tube duration (d) | |
| Range | 2–10 |
| Median | 4 |
| Postoperative hospital stay (d) | |
| Range | 2–13 |
| Median | 4 |
| Postoperative complications | |
| Prolonged air leakage (>5 d) | 5 |
| Prolonged pneumonia and liver dysfunction | 1 |
| Tumor size (cm) | |
| Range | 0.2–1.5 |
| Median | 0.8 |
| Histologic subtypes | |
| AAH | 1 |
| MIA | 13 |
| IAC | 11 |
| Inflammatory nodules | 6 |
| Pathological TNM stage of lung cancer | |
| 0 | 1 |
| IA1 | 20 |
| IA2 | 4 |
AAH, atypical adenomatous hyperplasia; MIA, minimally invasive; IAC, invasive adenocarcinoma; TNM, Tumor Node Metastasis classification.
Discussion
Although lobectomy plus mediastinal lymphadenectomy is still the gold standard for the surgical treatment of lung cancer (15), increasing evidence shows that segmental resection (16-18) can achieve the same radical effect for partial early lung cancer. Compared with lobectomy, segmental resection requires higher surgical skills and anatomical structure identification.
Basal segmental resection is a difficult stage during segmental resection of the lung, especially resection of the posterior and/or lateral basal segments. The main reason for this is that the portal structure of the posterior and/or lateral basal segments penetrate deep into the lung parenchyma and varies greatly. Through the accumulation of experience in early simple lung segment surgery, we carried out thoracoscopic dissection of the posterior and/or lateral basal segments by employing the inferior pulmonary ligament approach and/or interlobar fissure approach. From January 2020 to June 2022, a total of 31 patients have undergone this surgical procedure successfully, achieving good results and no deaths.
Due to the deep position and lack of pleural shrinkage, it is difficult to accurately detect small pulmonary nodules in the posterior or lateral basal segments using the naked eye and experience alone, and lobectomy may even be needed because of incomplete lung segment resection. In this group, there was one intersegmental nodule in right segment (RS) 9. Following RS10+9 resection, the surgical margin did not meet the requirements, and the right lower lobe required enlargement. Therefore, it is necessary to carefully determine the type of anatomical structure, including whether there are any variations or deformities, etc. Preoperative planning should be performed according to the size, location, imaging diagnosis, and reliable incisal edge of the lesion based on thin-layer CT and 3D-CTBA (19-21). Moreover, the designed resection sequence should follow the principle of “from shallow to deep” during the operation. The surgeon should try to move to the distal hilum, preferably to the subsegmental branch, after the dissecting section hilum, carefully identify the anatomical structure of the target segment, compare it with the preoperative images, observe whether it is consistent, and then implement the procedure according to the preoperative operation plan.
There are two common surgical methods of posterior or lateral basal segment resection, each with its own characteristics. The characteristics of the traditional interlobar fissure approach are as follows: the exposure is clear and the judgment is relatively simple. However, this method requires the separation of the space between the dorsal and basal segments, and the operation is complicated. Furthermore, the surgical wound is large and the operation takes a long time. Following resection of the posterior lateral basal segment, the dorsal and anterior inner basal segments are separated, and thus, it is necessary to suture the dorsal and anterior basal segments again to avoid the risk of torsion.
The features of the unidirectional inferior pulmonary ligament approach are as follows: relatively simple operation, easy to master, no need to separate the lung tissue intraoperatively between the dorsal and basal segments, as well as a small wound and short operative time, thereby avoiding the risk of pulmonary torsion postoperatively, and ensuring high operator fluency and comfort. However, this method requires more of the surgeon. It is necessary to have a very clear identification of the preoperative imaging interpretation and intraoperative portal structure as well as higher surgical skills.
Therefore, we adopted the idea of mainly passing through the inferior pulmonary ligament, supplemented by the interlobar fissure approach, and performed posterior and/or lateral basal segment resection. Since the variation in the segmental bronchus is relatively small, we usually take the bronchus as the center as a reliable intraoperative reference. Typically, the collateral relationship between the artery and bronchus at the segmental hilum is not very close, but at the subsegmental branch, the artery and bronchus are close. Therefore, the length must be sufficient, regardless of the free vein, bronchus, or artery. On the one hand, they can be compared with each other for identification, and on the other hand, tearing and bleeding of the pulmonary vessels during ligation or treatment with cutting and suturing devices can be avoided. Two patients in this study cohort underwent surgery via the interlobar fissure approach, mainly in the early stage. As it is not possible to form a one-to-one correspondence between each structure of the portal and preoperative CT and 3D reconstruction based on the preoperative planning, this method is used for comparison and confirmation to avoid false or missing data. However, when the anatomical results were familiar, we routinely applied the approach through the inferior pulmonary ligament to perform this kind of procedure, and 25 cases in this group utilized only this method to complete the operation. Yet, if the doornail lymph nodes are encountered during the operation or it is difficult to identify them, further confirmation, separation, and segmentation can be carried out simultaneously with the inferior pulmonary ligament and interlobar fissure approach. If necessary, the hilum structure of the sections can be separated sequentially from bottom to top according to the order of “posterior lateral basal segment resection—lateral basal segment resection”. Four patients in this group underwent surgery by combining the two methods due to the above reasons.
Through the study, familiarity, and mastery of this operation, we have realized that there are two main difficulties in the resection of the posterior and/or lateral basal segment. First, it is difficult to confirm the vein of the posterior lateral basal segment. The main vein in the posterior and/ or lateral basal segment varies greatly. With the exception of the posterior lateral basal segment vein (V10), which is usually the lowest branch, the other pulmonary veins often have distant branches. There are intersegmental veins between the adjacent lung segments, and a reticular structure is formed between the lung segments. Therefore, it is necessary to free the basilar segment vein to the distal end of the lung as far as possible during the operation and judge the vein to which the posterior outer basilar segment belongs according to the course of the vein. The better cut less than cut more principle should be applied. With unclear discrimination, if it does not affect the processing of the intersegment plane, it can be continued; surgeons should try to cut off only the intrasegmental vein and keep the intersegmental vein. All 20 patients in this cohort underwent chest plain film or chest CT examination on the 2nd day postoperatively, and no obvious pulmonary congestion was observed. There was no hemoptysis at 1 week postoperatively.
Second, it is very difficult to judge the plane between segments. The traditional method involves using the expansion and collapse approach (22), which is inaccurate. When the residual lung is expanded, the gas of adjacent lung segments will diffuse to the target segment, and the target segment will also expand as a result. At this point, it becomes difficult to accurately judge the boundary between lung segments. Therefore, we adopted the “Improved Expansion and Collapse Method” (14) to accurately define the boundary between lung segments and improve the process of expansion and collapse. This showed that the boundary line of expansion and collapse is clear and distinguishable, which has the advantages of accuracy, safety, simplicity, good repeatability, and no complications, and can be applied to all kinds of lung resections. After exposing the intersegmental plane, guided by the junction of the intersegmental vein and expansion and collapse, the intersegmental plane was processed by sharp separation and suture separation, and the techniques of dimension reduction and door opening were comprehensively applied (23,24). The anatomical fault and leakage will affect the precise definition of the interface between lung segments, and it has been determined that the leakage needs to be cut off and the surgical scheme needs to be changed when the main blood vessels or bronchi of adjacent segments are broken by mistake.
In this cohort of patients, two cases were treated using the simple interlobar fissure approach, 25 cases by the inferior pulmonary ligament approach, and four cases by a combination of the two methods. All patients successfully completed the operation without serious intraoperative events or complications, and there were no perioperative deaths. Therefore, a full understanding of preoperative thin-slice CT and/or 3D-CTBA by follow-up practitioners and the gradual accuracy of understanding the anatomical structure, with the continuous improvement of endoscopic operation skills, is safe and feasible to master thoracoscopic resection and/or lateral basal segment resection via the inferior pulmonary ligament approach. This approach is worthy of clinical promotion for the following reasons: it is a simpler operation, less time-consuming, creates a smaller wound surface, provides better protection of lung tissue, the whole operation process is more reasonable, the efficiency is higher, and it offers greater benefits for patients. If there is any difficulty in judgment during the operation, the two methods can be comprehensively selected according to the situation to avoid irreparable damage to patients.
Conclusions
The approach of the inferior pulmonary ligament to resect posterior and/or the lateral basal segment can optimize the surgical procedure. The surgical trauma and postoperative complications are reduced, which is worthy of popularization and application.
Acknowledgments
Funding: The study was supported by the Science& Technology Department of Sichuan Province (No. 2021YFG0321, to Gang Li).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1719/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1719/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1719/coif). GL reports that the study was supported by the Science& Technology Department of Sichuan Province (2021YFG0321). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), The study was approved by Ethics Committee of Sichuan Provincial People’s Hospital (No. 2021-516). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Goudemant C, Durieux V, Grigoriu B, et al. Lung cancer screening with low dose computed tomography: a systematic review. Rev Mal Respir 2021;38:489-505. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Krueger T, et al. Clinical outcome and risk factors for complications after pulmonary segmentectomy by video-assisted thoracoscopic surgery: results of an initial experience. J Thorac Dis 2018;10:5023-9. [Crossref] [PubMed]
- Chen L, Wang J, Wu W, et al. Technical process and quality control of precise thoracoscopic lung segmentectomy. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2019;26:21-8.
- Kikkawa T, Kanzaki M, Isaka T, et al. Complete thoracoscopic S9 or S10 segmentectomy through a pulmonary ligament approach. J Thorac Cardiovasc Surg 2015;149:937-9. [Crossref] [PubMed]
- Pu Q, Liu C, Guo C, et al. Stem-Branch: A Novel Method for Tracking the Anatomy During Thoracoscopic S9-10 Segmentectomy. Ann Thorac Surg 2019;108:e333-5. [Crossref] [PubMed]
- Liu C, Liao H, Guo C, et al. Single-direction thoracoscopic basal segmentectomy. J Thorac Cardiovasc Surg 2020;160:1586-94. [Crossref] [PubMed]
- Guo C, Liu C, Lin F, et al. Intrathoracic vertical overhanging approach for placement of an endo-stapler during single-port video-assisted thoracoscopic lobectomy†. Eur J Cardiothorac Surg 2016;49:i84-i86. [Crossref] [PubMed]
- Lin Z, Jiang W, Wang L, et al. Surgical techniques of thoracoscopic basal segment resection. Chin J Thorac Surg (Electronic Edition) 2016;3:125-6.
- Sato M, Murayama T, Nakajima J. Thoracoscopic stapler-based "bidirectional" segmentectomy for posterior basal segment (S10) and its variants. J Thorac Dis 2018;10:S1179-S1186. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Kawatani N, et al. Thoracoscopic lateral and posterior basal (S9 + 10) segmentectomy using intersegmental tunnelling. Eur J Cardiothorac Surg 2017;51:790-1. [Crossref] [PubMed]
- Wang J, Xu X, Wen W, et al. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac Cancer 2018;9:330-3. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Zhang Y, Fu F, Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann Thorac Surg 2020;110:1796-804. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Iwano S, Usami N, Yokoi K, et al. Segmentectomy simulation using a virtual three-dimensional safety margin. Ann Thorac Surg 2012;93:e37-9. [Crossref] [PubMed]
- Xu G, Du J, Chen C, et al. Intersegmental plane simulation based on the bronchus-vein-artery triad in pulmonary segmentectomy. Transl Cancer Res 2021;10:4702-13. [Crossref] [PubMed]
- Sardari Nia P, Olsthoorn JR, Heuts S, et al. Interactive 3D Reconstruction of Pulmonary Anatomy for Preoperative Planning, Virtual Simulation, and Intraoperative Guiding in Video-Assisted Thoracoscopic Lung Surgery. Innovations (Phila) 2019;14:17-26. [Crossref] [PubMed]
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg 1939;109:481-99. [Crossref] [PubMed]
- Wang J, Xu X, Wen W, et al. Technique for tailoring complex demarcation in lung segmentectomy. Thorac Cancer 2018;9:1562-4. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-5. [Crossref] [PubMed]
(English Language Editor: A. Kaseem)