Preoperative albumin corrected anion gap is associated with in-hospital and long-term mortality in patients undergoing coronary artery bypass grafting in a retrospective cohort study
Highlight box
Key findings
• In this paper, we found that preoperative ACAG is an independent risk factor for in-hospital and long-term mortality in CABG patients.
What is known and what is new?
• ACAG was associated with the severity of disease and prediction of mortality in critical patients. In coronary heart disease, severe coronary artery stenosis and heart failure might lead to ACAG level increased.
• CABG patients with higher ACAG tend to had more comorbidities which increased the risk of death.
What is the implication, and what should change now?
• We can calculate ACAG to assess the risk of death in patients who underwent CABG, which may give some advises on the following treatment. And in multivessel coronary artery disease, it may help us choice the operation methods.
Introduction
Coronary artery disease remains a global health concern and the leading cause of death (1,2). The NHANES (National Health and Nutrition Examination Survey) reported that in the United States 18.2 million people over the age of 20 years suffered from coronary diseases with a prevalence of 6.7% (1), and approximately one in forty individuals had an acute cardiovascular event requiring further treatment (1). According to a WHO annual report, 17.9 million people die of cardiovascular disease annually, accounting for 32% of global deaths (2). Coronary artery bypass grafting (CABG) is one of the two main treatment strategies for coronary artery disease (CAD), and the other one is percutaneous coronary interventions (PCI). Since the 1990s, the use of PCI for coronary artery disease is on the rise. While CABG was more dominant than PCI in patients with complex CAD , particularly in patients with multivessel coronary artery disease or diabetes (3). As we all known, multivessel CAD is more complicated than others, which usually means a worse prognosis. The prognosis of patients undergoing CABG is related to multiple factors, including age, gender, body mass index (BMI), the severity of coronary artery stenosis, and comorbidities (3-5). Risk factors identified preoperatively for mortality may facilitate risk stratification of CABG patients to improve their prognosis.
Anion gap (AG) is defined as the concentration gradient between the negative and positive ions [AG (mmol/L) = (sodium + potassium) − (chloride + bicarbonate)] (6-8). It is an important indicator to evaluate the acid-base balance of body fluids and classify the type of metabolic acidosis. However, AG is highly related to serum albumin level, and it is reported that every 1 g/dL decrease in serum albumin is associated with a corresponding decrease in AG at 2.5 mmol/L (6,8-10). Therefore, the albumin corrected anion gap (ACAG) was created to avoid the underestimation of AG in hypoalbuminemia, which is common among cardiac patients. This sees 2.5 mmol of negative ions added for every 1 g/dL decrease in albumin as follows: [4.5 g/dL − serum albumin (g/dL)] × 2.5 (6,8). ACAG has been identified as an independent risk factor for chronic renal insufficiency (11,12), sepsis (7,13), cardiac arrest (6), and acute myocardial infarction (6-8). In the present study, we aimed to investigate the relationship between ACAG and the prognosis of patients undergoing CABG. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1633/rc).
Methods
Patient selection
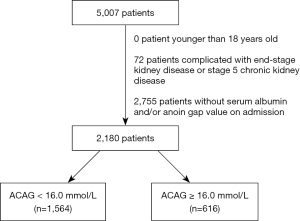
The present study was conducted based on the Medical Information Mart for Intensive Care III (MIMIC-III) database (v1.4). In this database, clinic data were collected from the Beth Israel Deaconess Medical Center (14). MIMIC-III data is available on the PhysioNet repository (15,16). We focused primarily on data from ICU patients who undergoing CABG. According to the date we extracted from MIMIC-III data. In intensive care unit (ICU), there were more than 40,000 patients between 2001 to 2012. In MIMIC-III data, those who underwent CABG were eligible for inclusion and identified using the ICD9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) code (3610-3616). There are patients with multiple hospital admission, we only included their first admission for CABG. They formed a consecutive, random or convenience series. The exclusion criteria were as follows: (I) patients <18 years old; (II) patients complicated with end-stage kidney disease (ESKD) (ICD9-CM code: 5855 and 5856); (III) patients missing albumin and/or AG value on admission. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variable extraction
The clinical data were collected using structured query language. Authors (RW, JL) extracted meaningful data from MIMIC-III database. We collected demographics of patients like age, gender and body mass index (BMI) as well as their length of stay in ICU. We also collected their laboratory examinations on admission, likealbumin, AG, hemoglobin, creatinine, bilirubin, white blood cell count, platelet count and blood urea nitrogen (BUN). As Quan et al. suggested (17), we identified the comorbidities using the ICD9-CM code, including congestive heart failure, cardiac arrhythmias, chronic pulmonary diseases, myocardial infarction, liver diseases, renal diseases, peripheral vascular diseases and diabetes mellitus. Thereby, the Charlson comorbidity index (CCI) was calculated. In addition, the concomitant surgeries including cardiopulmonary bypass (CPB), aortic surgery and valvular surgery were also collected. We used the ICD9-CM code to identify the number of revascularized coronary artery and internal mammary artery (IMA) used for bypass grafting. The in-hospital mortality and 4-year mortality were collected respectively, considering all dates of death up to 4 years for patients from CareVue system were contained in MIMIC-III database. ACAG was calculated with the following formula: ACAG (mmol/L) = AG (mmol/L) + [4.4 – albumin (g/dL)] ×2.5.
Outcome
The primary outcome in the present study was in-hospital mortality and the secondary outcome was 4-year mortality.
Statistical analysis
Data are presented as means (standard deviations) or medians (interquartile ranges) for continuous variables, and total numbers (percentages) for categorical variables. Receiver operating characteristic (ROC) curves were constructed and used to determine the optimal cutoff value of ACAG for in-hospital mortality Subsequently, patients were divided into High ACAG group and Low ACAG group. The Student-t test or Mann-Whitney U test were used for the comparison of continuous variables, and the Chi-square test or Fisher’s exact test were used for the comparison of for categorical variables, as appropriate The Kaplan-Meier method and log-rank test were used to estimate the survival curves. Logistic regression analysis and Cox regression analysis were used to determine the relationship of ACAG with mortality. We constructed different models to explore the confounding effect of covariates on the relationship between ACAG and mortality. We constructed model 1 to adjusted for demographics like gender, age and BMI. And in order to adjusted for demographics, laboratory examinations on admission, comorbidities, and CCI, we constructed model 2. The last one, model 3 was adjusted for concomitant surgeries and all of those variables in model 2. We analysed the 4-year mortality, but it was restricted to patients in the CareVue system.
All tests were two-sided, and P<0.05 were considered significant. All statistical analyses in our study were performed using SPSS Statistics 23 (IBM, Chicago, IL, USA).
Results
Baseline characteristics
The flow diagram of patient selection is presented in Figure 1. Totally, 2,180 CABG patients were identified and included, and their clinical characteristics are presented in Table 1. The median of ACAG on admission was 14.63 (13.25, 14.63) mmol/L, with a minimum value of 8.0 and maximum value of 26.1. Among 1,338 patients in the CareVue system, the in-hospital mortality was 1.8% and the 4-year mortality was 17.6%.
Table 1
| Characteristics | All (n=2,180) |
|---|---|
| Demographics | |
| Age | 68.89 (61.17, 76.95) |
| Gender | |
| Male | 1,594 (73.1) |
| Female | 586 (26.9) |
| BMI | 27.95 (24.87, 31.54) |
| Comorbidities | |
| Cardiac arrhythmia | 1,084 (49.7) |
| Myocardial infarction | 931 (42.7) |
| Congestive heart failure | 663 (30.4) |
| Peripheral vascular disease | 315 (14.4) |
| Chronic pulmonary disease | 401 (18.4) |
| Liver disease | 69 (3.2) |
| Diabetes mellitus | 789 (36.2) |
| Kidney disease | 201 (9.2) |
| CCI | 2.00 (1.00, 3.00) |
| Laboratory examinations* | |
| Albumin (g/dL) | 3.80 (3.60, 4.10) |
| AG (mmol/L) | 13.00 (12.00, 15.00) |
| ACAG (mmol/L) | 14.63 (13.25, 14.63) |
| Bilirubin (mg/dL) | 0.50 (0.40, 0.70) |
| Hemoglobin (g/dL) | 11.99 (10.70, 13.40) |
| White blood cell (×109/L) | 8.30 (6.70, 10.60) |
| Platelet (×109/L) | 209.00 (171.00, 256.00) |
| Creatinine (mg/dL) | 0.97 (0.80, 1.20) |
| BUN (mg/dL) | 18.00 (14.00, 23.17) |
| Concomitant surgeries | |
| CPB | 2042 (93.7) |
| Valvular surgery | 399 (18.3) |
| Mitral valve surgery | 135 (6.2) |
| Aortic valve surgery | 292 (13.4) |
| Pulmonary valve surgery | 1 (0.0) |
| Tricuspid valve surgery | 3 (0.1) |
| Aortic surgery | 18 (0.8) |
| Revascularized arteries& | 2.00 (2.00, 3.00) |
| 1 | 463 (21.2) |
| 2 | 857 (39.3) |
| 3 | 665 (30.5) |
| ≥4 | 195 (8.9) |
| IMA&& | 1.00 (1.00, 1.00) |
| 0 | 230 (10.6) |
| 1 | 1,930 (88.5) |
| 2 | 20 (0.9) |
| Outcome | |
| Length of stay in ICU | 2.83 (1.34, 4.65) |
| In-hospital mortality | 39 (1.8) |
| 4-year mortality† | 236 (17.6) |
Data are presented as means (standard deviations) or medians (interquartile ranges) for continuous variables, and total numbers (percentages) for categorical variables. *, laboratory examinations on admission; &, number of revascularized coronary arteries; &&, number of IMA used for bypass grafting; †, limited to patients in the CareVue system. ACAG, albumin correct anion gap; AG, anion gap; BMI, body mass index; BUN, Blood urea nitrogen; CABG, Coronary artery bypass grafting; CCI, Charlson comorbidity index; CPB, cardiopulmonary bypass; ICU, intensive care unit; IMA, internal mammary artery.
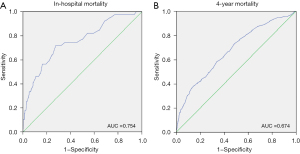
As shown in Figure 2, ROC analysis was showed to evaluate the predictive value of ACAG for in-hospital mortality, and the area under the curve (AUC) was 0.754 (P<0.001). The optimal cutoff value of ACAG for in-hospital mortality was 16.0 mmol/L with a sensitivity of 0.718 and a specificity of 0.725. However, the discriminatory ability of ACAG for 4-year mortality was unsatisfactory (AUC: 0.674, P<0.001).
Patients were divided into two groups according to the level of ACAG on admission: A high ACAG group (ACAG ≥16.0 mmol/L; n=616) and low ACAG group (ACAG <16.0 mmol/L; n=1,546). In addition, 461 (74.8%) patients in the high ACAG group and 877 (56.1%) patients in the low ACAG group. The baseline characteristics of the two groups were significantly different (Table 2). In the high ACAG group, patients had the tendency to be older and female made up a larger proportion. We observed a significantly higher level of AG, white blood cell count, platelets, BUN and creatinine on admission, but lower levels of albumin and hemoglobin. Patients in the high ACAG group also tended to have more comorbidities including cardiac arrhythmia, myocardial infarction, congestive heart failure, chronic pulmonary disease, liver disease, diabetes mellitus and diabetes mellitus. Concomitant valvular surgeries were more likely to be performed in the high ACAG group.
Table 2
| Characteristics | Low ACAG group (n=1,564) |
High ACAG group (n=616) |
P |
|---|---|---|---|
| Demographics | |||
| Age | 68.26 (60.44, 76.43) | 70.97 (63.45, 78.05) | <0.001 |
| Gender | <0.001 | ||
| Male | 1,203 (76.9) | 391 (63.5) | |
| Female | 361 (23.1) | 225 (36.5) | |
| BMI | 27.67 (24.91, 31.41) | 28.26 (24.73, 31.91) | 0.196 |
| Comorbidities | |||
| Cardiac arrhythmia | 738 (47.2) | 346 (56.2) | <0.001 |
| Myocardial infarction | 591 (37.8) | 340 (55.2) | <0.001 |
| Congestive heart failure | 358 (22.9) | 305 (49.5) | <0.001 |
| Peripheral vascular disease | 223 (14.3) | 92 (14.9) | 0.686 |
| Chronic pulmonary disease | 253 (16.2) | 148 (24.0) | <0.001 |
| Liver disease | 40 (2.6) | 29 (4.7) | 0.010 |
| Diabetes mellitus | 513 (32.8) | 276 (44.8) | <0.001 |
| Kidney disease | 102 (6.5) | 99 (16.1) | <0.001 |
| CCI | 1.00 (1.00, 2.00) | 2.00 (1.00, 4.00) | <0.001 |
| Laboratory examination* | |||
| Albumin (g/dL) | 3.90 (3.70, 4.10) | 3.60 (3.30, 3.90) | <0.001 |
| AG | 12.50 (11.50, 13.50) | 15.50 (14.67, 16.67) | <0.001 |
| ACAG | 13.92 (12.75, 14.75) | 17.25 (16.50, 18.50) | <0.001 |
| Bilirubin (mg/dL) | 0.50 (0.40, 0.70) | 0.60 (0.40, 0.70) | 0.302 |
| Hemoglobin (g/dL) | 12.15 (10.90, 13.53) | 11.70 (10.35, 13.00) | <0.001 |
| White blood cell (×109/L) | 7.90 (6.50, 10.05) | 9.40 (7.47, 11.88) | <0.001 |
| Platelet (×109/L) | 204.50 (167.00, 250.00) | 224.00 (180.95, 275.08) | <0.001 |
| Creatinine (mg/dL) | 0.93 (0.80, 1.10) | 1.10 (0.87, 1.40) | <0.001 |
| BUN (mg/dL) | 17.00 (13.67, 21.33) | 21.00 (16.50, 31.33) | <0.001 |
| Concomitant surgeries | |||
| CPB | 1,466 (93.7) | 576 (93.5) | 0.844 |
| Valvular surgery | 262 (16.8) | 137 (22.2) | 0.003 |
| Mitral valve surgery | 80 (5.1) | 55 (8.9) | |
| Aortic valve surgery | 196 (12.5) | 96 (15.6) | |
| Pulmonary valve surgery | 1 (0.1) | 0 | |
| Tricuspid valve surgery | 1 (0.1) | 2 (0.3) | |
| Aortic surgery | 10 (0.6) | 8 (1.3) | 0.126 |
| Revascularized arteries& | 2.00 (2.00, 3.00) | 2.00 (2.00, 3.00) | 0.625 |
| 1 | 322 (20.6) | 141 (22.9) | |
| 2 | 623 (39.8) | 234 (38.0) | |
| 3 | 484 (30.9) | 181 (29.4) | |
| ≥4 | 135 (8.6) | 60 (9.7) | |
| IMA&& | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | <0.001 |
| 0 | 141 (9.0) | 89 (14.4) | |
| 1 | 1405 (89.8) | 525 (85.2) | |
| 2 | 18 (1.2) | 2 (0.3) | |
| Outcome | |||
| Length of stay in ICU | 2.29 (1.29, 3.94) | 3.88 (2.15, 7.09) | <0.001 |
| In-hospital mortality | 11 (0.7) | 28 (4.5) | <0.001 |
| 4-year mortality† | 111 (12.7) | 125 (27.1) | <0.001 |
For continuous variables, data are reported as means (standard deviations) or medians (interquartile ranges), and categorical variables are reported as total numbers (percentages). *, Laboratory examinations on admission; &, Number of revascularized coronary arteries; &&, Number of IMA used for bypass grafting; †, Limited to patients in the CareVue system. ACAG, albumin correct anion gap; AG, anion gap; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CCI, Charlson comorbidity index; CPB, cardiopulmonary bypass; ICU, intensive care unit; IMA, internal mammary artery.
Outcome
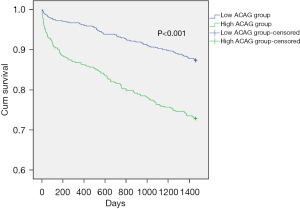
As shown in Table 2, patients in the high ACAG group had a longer length of stay in ICU [3.88 (2.15, 7.09) vs. 2.29 (1.29, 3.94), P<0.001]. And in comparison with those in the low ACAG group, they had higher in-hospital mortality [28 (4.5%) vs. 11 (0.7%), P<0.001] and higher 4-year mortality [125 (27.1%) vs. 111 (12.7%), P<0.001]. We used the Kaplan-Meier survival curves to make the comparison of the 4-year mortality between two groups. And it was shown in Figure 3. In the high ACAG group, we also observed significantly lower 4-year survival (P<0.001).
In Figure 4, we showed the curvilinear relationship between ACAG and mortality. The incidence of in-hospital mortality dramatically rised as ACAG up to approximately 16 mmol/L, while the incidence of 4-year mortality increased as ACAG increased. After full adjustment, the logistic regression models showed ACAG was an independent risk factor for in-hospital mortality [OR: 1.248 (1.060, 1.470), P=0.008; Table 3], and an ACAG ≥16.0 mmol/L was significantly associated with a 2.7-fold risk of in-hospital mortality [OR: 2.732 (1.129, 6.610), P=0.026]. The Cox regression models indicated ACAG was independently associated with 4-year mortality [HR: 1.134 (1.063, 1.210), P<0.001], but an ACAG above 16.0 mmol/L was not significantly associated [HR: 1.198 (0.873, 1.643), P=0.263].
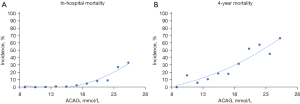
Table 3
| Outcome | Variables | OR/HR (95% CI) | |||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | ||
| In-hospital mortality | ACAG (mmol/L) | 1.441 (1.300, 1.598) | 1.427 (1.278, 1.595) | 1.238 (1.050, 1.458) | 1.248 (1.060, 1.470) |
| ACAG ≥16.0 mmol/L | 6.723 (3.326, 13.591) | 5.725 (2.784, 11.772) | 2.892 (1.209, 6.914) | 2.732 (1.129, 6.610) | |
| 4-year mortality† | ACAG (mmol/L) | 1.261 (1.206, 1.319) | 1.246 (1.189, 1.305) | 1.126 (1.055, 1.201) | 1.134 (1.063, 1.210) |
| ACAG ≥16.0 mmol/L | 2.391 (1.851, 3.087) | 2.141 (1.649, 2.779) | 1.203 (0.877, 1.652) | 1.198 (0.873, 1.643) | |
Model 1 adjusted for age, gender, BMI. Model 2 adjusted for variables in model 1, plus comorbidities (congestive heart failure, myocardial infarction, cardiac arrhythmias, peripheral vascular disease, liver disease, chronic pulmonary disease, renal disease, and diabetes mellitus), CCI and laboratory examinations (bilirubin, white blood cells, platelets, BUN, creatinine, and hemoglobin) on admission. Model 3 adjusted for variables in model 2, plus concomitant surgeries (CPB, valvular surgery, aortic surgery, and number of revascularized coronary artery and number of IMA used for bypass grafting). †, Limited to patients in the CareVue system. ACAG, albumin correct anion gap; OR, odds ratio; HR, hazard ratio; BMI, body mass index; CCI, Charlson comorbidity index; BUN, blood urea nitrogen; CPB, cardiopulmonary bypass; IMA, internal mammary artery.
Discussion
In the present study, we investigated the association of preoperative ACAG with mortality in patients undergoing CABG. Our results showed ACAG was independently associated with a higher risk of in-hospital and 4-year mortality.
AG is an indicator in clinical practice to assess the type of acid-base imbalance, and a high AG level usually indicates the presence of metabolic acidosis. Elevated AG levels have been proven to be associated with hypertension, insulin resistance, and cardiopulmonary dysfunction (7,8), However, an underestimated level of AG was observed in patients with liver cirrhosis, gastrointestinal bleeding, hypoproteinemia, and multiple myeloma owing to the reduction albumin level (9,10). Consequently, ACAG was created to avoid the negative impact of hypoalbuminemia (10-12), which is common among patients with coronary artery disease (6,8).
Previous studies found elevated ACAG was associated with the severity of disease and prediction of mortality (13,17-19). Hu et al. found a strong correlation between ACAG and 1-year mortality in critical patients with sepsis (13), which might be due to the abnormal energy metabolism caused by a systemic inflammatory response and subsequent microcirculation disorder and lactic acid accumulation. It was suggested the accelerated protein decomposition and accumulation of acidic metabolites could lead to increased ACAG in patients with a hypermetabolic state (18,20). In addition, the accumulation of acidic products due to anaerobic glycolysis can increase the level of ACAG (13,18). ACAG can be used to evaluate the metabolic state (13,20) and as an indicator for the selection of treatment in patients with chronic obstructive pulmonary disease, pulmonary heart disease, and diabetic ketoacidosis (11). In the present study, the increase of ACAG preoperatively may be related to the accumulation of metabolites and lactic acid caused by microcirculation disorders, which are associated with poor prognosis.
Lazzeri et al. found severe coronary artery stenosis and heart failure might lead to the accumulation of serum lactic acid and ketone bodies by accelerating basal metabolic rates, activation of sympathetic nerves, and increased anaerobic glycolysis, which could result in an increased ACAG level (21). It was also shown that high levels of ketone bodies and the accumulation of organic acid could elevate ACAG level in patients with cardiac insufficiency (22,23). The association between ACAG and the prognosis of patients with coronary heart disease has been reported. In a large multicenter Chinese study involving 18,115 patients, the serum ACAG level in patients with coronary heart disease was significantly higher than in the general population (24). It was reported that serum ACAG levels were independently related to the severity of coronary artery stenosis and all-cause mortality in patients with coronary heart disease (24,25). Based on the analysis of patients in the cardiology-ICU, Sun et al. found serum ACAG levels positively correlated with the length of hospital stay and SOFA (Sequential Organ Failure Assessment) score, and were independently predictive of all-cause mortality (26). However, the association of ACAG with the prognosis of patients undergoing CABG has not been investigated. Our results showed elevated ACAG was independently associated with mortality in CABG patients, and an ACAG ≥16.0 mmol/L indicated a 2.7-fold risk of in-hospital mortality. The high ACAG level on admission may indicate more severe coronary artery stenosis and cardiac dysfunction.
In critical patients, ACAG, as a metabolic index, can be used as a diagnostic and prognostic indicator for monitoring resuscitation, together with serum bicarbonate level, base excess, mixed venous oxygen saturation, and central venous oxygen saturation (8,27,28). He et al. suggested that in patients with sepsis and multiple organ dysfunction, those with a higher ACAG were more likely to be exposed to renal insufficiency or intestinal dysfunction as compared to those with normal ACAG (29). In patients with chronic kidney disease (CKD), serum AG levels could increase significantly due to the accumulation of metabolites, and higher levels only appear in patients with stage 5 disease (CKD 5, eGFR <30 mL/min/1.73 m2). Recently, ACAG was shown to exhibit a gradual upward trend with the progression of CKD and association with all-cause mortality (25) despite the level of eGFR and proteinuria. In the present study, hemoglobin was lower in patients with higher ACAG, suggesting an increased need for transfusion during extracorporeal circulation and compromised oxygen supply leading to microcirculation disorders and increased lactate accumulation. In the present study, patients in the high ACAG group tended to be older and of higher BMI, and had more comorbidities including cardiac arrhythmia, myocardial infarction, congestive heart failure, chronic pulmonary disease, liver disease, diabetes mellitus, and renal disease, which increased the risk of death.
Limitations
Several limitations in the present study should be taken into consideration. Firstly, as this is a retrospective study, variables like cardiac troponin natriuretic peptide and left ventricular ejection fraction, which are closely related to coronary artery disease, were not available. Secondly, variables of perfusion time and aortic clamping time related to the prognosis of CABG were not available in the MIMIC-III database. Future study may take these variables into account to further investigate the association of ACAG with mortality.
Conclusions
Preoperative ACAG is an independent risk factor for in-hospital and long-term mortality in CABG patients. A higher ACAG may relate to severe coronary artery stenosis and cardiac dysfunction, which is more likely to lead to a postoperative systemic inflammatory response, microcirculation disorder, and subsequent complications. However, the mechanism requires further verification.
Acknowledgments
Funding: This research was supported by grants from the National Natural Science Foundation of China (No. 82170274), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515011747), Guangzhou Key Research & Development Program (No. 202206010065), and Guangdong Provincial International Science and Technology Cooperation Project (No. 2022A0505050036).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1633/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1633/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153-639. [Crossref] [PubMed]
- World Health Organization. Cardiovascular disease 2017. Available online: http://www.who.int/cardiovascular_diseases/en/, 2017.
- Rocha RV, Wang X, Fremes SE, et al. Variations in Coronary Revascularization Practices and Their Effect on Long-Term Outcomes. J Am Heart Assoc 2022;11:e022770. [Crossref] [PubMed]
- McNichols B, Spratt JR, George J, et al. Coronary Artery Bypass: Review of Surgical Techniques and Impact on Long-Term Revascularization Outcomes. Cardiol Ther 2021;10:89-109. [Crossref] [PubMed]
- Meharwal ZS, Trehan N. Off-pump coronary artery bypass grafting in patients with left ventricular dysfunction. Heart Surg Forum 2002;5:41-5.
- Kaneko M, Hagiwara S, Aoki M, et al. The Significance of Strong Ion Gap for Predicting Return of Spontaneous Circulation in Patients with Cardiopulmonary Arrest. Open Med (Wars) 2017;12:33-8. [Crossref] [PubMed]
- Zampieri FG, Park M, Ranzani OT, et al. Anion gap corrected for albumin, phosphate and lactate is a good predictor of strong ion gap in critically ill patients: a nested cohort study. Rev Bras Ter Intensiva 2013;25:205-11. [Crossref] [PubMed]
- Figge J, Rossing TH, Fencl V. The role of serum proteins in acid-base equilibria. J Lab Clin Med 1991;117:453-67.
- Chioléro RL, Revelly JP, Leverve X, et al. Effects of cardiogenic shock on lactate and glucose metabolism after heart surgery. Crit Care Med 2000;28:3784-91. [Crossref] [PubMed]
- Nanji AA, Campbell DJ, Pudek MR. Decreased anion gap associated with hypoalbuminemia and polyclonal gammopathy. JAMA 1981;246:859-60.
- Eustace JA, Astor B, Muntner PM, et al. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 2004;65:1031-40. [Crossref] [PubMed]
- Hsu CY, Chertow GM. Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant 2002;17:1419-25. [Crossref] [PubMed]
- Hu T, Zhang Z, Jiang Y. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: A retrospective propensity score matching analysis. Clin Chim Acta 2021;521:272-7. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Johnson A, Pollard T, Mark R. 2020. MIMIC-III Clinical Database. PhysioNet.
- Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000;101:E215-E220. [Crossref] [PubMed]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [Crossref] [PubMed]
- Kotake Y. Unmeasured anions and mortality in critically ill patients in 2016. J Intensive Care 2016;4:45. [Crossref] [PubMed]
- Lee SH, Park S, Lee JW, et al. The Anion Gap is a Predictive Clinical Marker for Death in Patients with Acute Pesticide Intoxication. J Korean Med Sci 2016;31:1150-9. [Crossref] [PubMed]
- Kraut JA, Madias NE. Treatment of acute metabolic acidosis: a pathophysiologic approach. Nat Rev Nephrol 2012;8:589-601. [Crossref] [PubMed]
- Lazzeri C, Valente S, Chiostri M, et al. Clinical significance of lactate in acute cardiac patients. World J Cardiol 2015;7:483-9. [Crossref] [PubMed]
- Lommi J, Kupari M, Koskinen P, et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 1996;28:665-72. [Crossref] [PubMed]
- Bedi KC Jr, Snyder NW, Brandimarto J, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016;133:706-16. [Crossref] [PubMed]
- Yang SW, Zhou YJ, Zhao YX, et al. The serum anion gap is associated with disease severity and all-cause mortality in coronary artery disease. J Geriatr Cardiol 2017;14:392-400. [Crossref] [PubMed]
- Abramowitz MK, Hostetter TH, Melamed ML. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int 2012;82:701-9. [Crossref] [PubMed]
- Sun T, Cai C, Shen H, et al. Anion Gap Was Associated with Inhospital Mortality and Adverse Clinical Outcomes of Coronary Care Unit Patients. Biomed Res Int 2020;2020:4598462. [Crossref] [PubMed]
- Gutierrez G, Wulf ME. Lactic acidosis in sepsis: another commentary. Crit Care Med 2005;33:2420-2. [Crossref] [PubMed]
- Chawla LS, Jagasia D, Abell LM, et al. Anion gap, anion gap corrected for albumin, and base deficit fail to accurately diagnose clinically significant hyperlactatemia in critically ill patients. J Intensive Care Med 2008;23:122-7. [Crossref] [PubMed]
- He X, Liao X, Xie Z, et al. Albumin corrected anion gap is an independent risk factor for long-term mortality of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017;29:117-21. [Crossref] [PubMed]
(English Language Editor: B. Draper)