
Efficacy and safety comparison of PD-1 inhibitors vs. PD-L1 inhibitors in extensive-stage small-cell lung cancer: a retrospective comparative cohort study
Highlight box
Key findings
• PD-1 and PD-L1 inhibitors provided comparable survival benefit in ES-SCLC patients.
What is known and what is new?
• PD-L1inhibitors atezolizumab, durvalumab and adebrelimab and PD-1 inhibitor serplulimab combined with EP were approved to be a standard treatment for ES-SCLC. But the evidence was not sufficient enough in some other PD-1 inhibitors.
• Our retrospective study suggested that PD-1 inhibitors could perform comparably with PD-L1 inhibitors in ES-SCLC treatment in clinical use.
What is the implication, and what should change now?
• PD-1 inhibitors included nivolumab, pembrolizumab, tislelizumab, sintilimab, toripalimab, and camrelizumab could be approved in clinical practice especially for patients hesitate about cost.
Introduction
Small-cell lung cancer (SCLC) is a high-grade malignant neuroendocrine tumor accounting for approximately 13–17% of all lung cancers (1). According to the Veterans’ Administration Lung Study Group (VALSG) staging system, SCLC is divided into limited-stage small cell lung cancer (LS-SCLC) and extensive-stage small cell lung cancer (ES-SCLC), with about 60% of patients classified as ES-SCLC when first diagnosed (1). The median survival rate for ES-SCLC patients receiving a standard chemotherapy regimen of platinum (cisplatin or carboplatin) combined with etoposide (EP) is 9–11 months (2).
EP regimen had been a standard first-line treatment for ES-SCLC until the participation of the immune-checkpoint inhibitors (ICIs) revealed better survival benefits (3,4). IMpower133 study obtained a significant improvement in terms of overall survival (OS) [12.3 vs. 10.3 months; hazard ratio (HR), 0.70; 95% confidence interval (CI), 0.54–0.91] and progression-free survival (PFS) (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62–0.96), and the ≥grade 3 AEs related to the trial regimen occurred in 58.1% patients. The CASPIAN study also demonstrated that durvalumab plus EP significantly improved OS (13.0 vs. 10.3 months; HR 0.73; 95% CI, 0.59–0.91), and grade 3 or 4 AEs occurred in 62% patients in the durvalumab group. Similar survival benefits were observed in the CAPSTONE-1 study (5). The KEYNOTE-604 and EA5161 studies of programmed cell death 1 (PD-1) inhibitors also revealed better OS and PFS (6,7), but the level of evidence was not sufficient. And according to the ASTRUM-005, Serplulimab demonstrated a longer median OS (mOS) of 15.4 months compared with 10.9 months in the placebo group, and ≥ grade 3 AEs occurred in 33.2% patients in the serplulimab group (8). Both PD-1 and programmed cell death ligand 1 (PD-L1) inhibitors revealed sufficient evidence of efficacy.
At present, a wide disparity existed between the cost for PD-L1 inhibitors and PD-1 inhibitors. The clinical researches of the comparation between the PD-1 and PD-L1 were lacking. Therefore, we carried out this retrospective cohort study to verify whether the two immunotherapy regimens were comparable. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1682/rc).
Methods
Patients
The data of 221 ES-SCLC patients treated with PD-1 or PD-L1 inhibitors between February 2017 and June 2020 at Shandong Cancer Hospital and Institute (Jinan, Shandong, China) were retrospectively collected. The inclusion criteria were as follows: (I) pathologically diagnosed SCLC; (II) staged ES-SCLC; (III) treated with PD-1 or PD-L1 inhibitors; (IV) with measurable lung lesions; (V) Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1; and (VI) with clear prognostic information. Exclusion criteria were: (I) patients with non-small cell lung cancer (NSCLC); (II) staged LS-SCLC; (III) uncontrolled, concurrent illness or active infections; (IV) patients who failed to be followed up (18 patients). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shandong Cancer Hospital (No. SDTHEC 2022011009). Individual consent for this retrospective analysis was waived.
Therapy
All ES-SCLC patients who had been treated with PD-1 or PD-L1 inhibitors were took into account. PD-1 inhibitors included nivolumab, pembrolizumab, tislelizumab, sintilimab, toripalimab, and camrelizumab, while PD-L1 inhibitors included durvalumab and atezolizumab. The combined chemotherapy regimens included etoposide combined with platinum, irinotecan combined with platinum, irinotecan, paclitaxel, docetaxel, and pemetrexed, among others. The anti-angiogenesis drug was mainly bevacizumab.
Endpoints and assessments
The primary endpoints were OS and PFS. OS was defined as the time interval from treatment initiation of PD-1/PD-L1 inhibitors to death caused by any reason or the last known follow-up. PFS was measured as the time interval from the initiation of PD-1/PD-L1 inhibitors to disease progression, death from any cause, or the last known follow-up. Assessment of treatment response was based on the modified response evaluation criteria in solid tumors (RECIST1.1) for immune based therapeutics (iRECIST). AEs as the secondary endpoint were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The AEs collected included neutrophil count decreased, white blood cell count decreased, platelet count decreased, anemia, nausea, vomiting, pneumonia and alanine/aspartate aminotransferase increased. The baseline clinical factors included gender, age, ECOG score, therapy, line of treatment, type of ICIs and combined with brain/liver/bone metastases or not.
Examinations for tumor assessment included computed tomography (CT) of the chest and abdomen, isotopic bone scan, and either a brain CT scan or magnetic resonance imaging (MRI). Positron emission tomography (PET) was encouraged but not necessary. The stage was defined according to the VALSG staging system.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software version 8.0 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS statistical software version 25.0 (SPSS, Inc., Chicago, IL, USA). The comparisons of patients’ baseline characteristics and AEs were analyzed by the Chi-square test and Fisher’s exact test. Kaplan-Meier methodology was used to estimate the OS and PFS for different groups. Univariate survival analysis was performed using the Kaplan-Meier methodology, and multivariate survival analysis was performed by Cox proportional hazards model to evaluate the independent prognostic factors associated with survival. Two-sided P values <0.05 were considered statistically significant. The missing data were deleted.
Results
Patients
The data of 221 ES-SCLC patients treated with PD-1 or PD-L1 inhibitors between February 2017 and June 2020 at Shandong Cancer Hospital and Institute (Jinan, Shandong, China) were retrospectively collected (Figure 1). The median age of patients was 62 years (range, 28–87 years), and there were 172 males (77.83%) and 49 females (22.17%). A total of 19 patients were treated with ICIs alone (8.60%), 20 patients were treated with ICIs plus anti-vascular endothelial growth factor (VEGF)/VEGF-receptor (VEGFR) agent (9.05%), 173 patients were treated with ICIs plus chemotherapy (78.28%), and 9 patients were treated with ICIs plus anti-VEGF/VEGFR agent and chemotherapy (4.07%). A total of 146 patients were treated with PD-1 inhibitors, while 75 received PD-L1 inhibitors, with 108 patients receiving immunotherapy as the first-line therapy (48.87%) and 113 as second-line or later therapy (51.13%). There were 82 patients with asymptomatic brain metastases included in the study (37.10%). The baseline clinical characteristics are shown in Table 1. The last follow-up was in July 2021, with a median follow-up time for all patients of 10.60 months (range, 0.27–34.17 months). The median follow-up time of surviving patients was 12.48 months (range, 7.37–34.17 months).
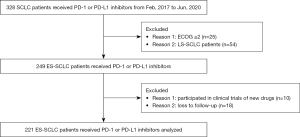
Table 1
| Clinical characteristics | Data, No. (%) |
|---|---|
| Gender | |
| Male | 172 (77.83) |
| Female | 49 (22.17) |
| Age, years | |
| Median | 62 |
| Range | 28–87 |
| ECOG | |
| 0 | 89 (40.27) |
| 1 | 132 (59.73) |
| Therapy | |
| ICIs | 19 (8.60) |
| ICIs + anti-VEGF/VEGFR agent | 20 (9.05) |
| ICIs + chemotherapy | 173 (78.28) |
| ICIs + anti-VEGF/VEGFR agent + chemotherapy | 9 (4.07) |
| Line | |
| First-line | 108 (48.87) |
| Second-line or later | 113 (51.13) |
| ICIs | |
| Nivolumab | 9 (4.07) |
| Pembrolizumab | 2 (0.90) |
| Tislelizumab | 24 (10.86) |
| Sintilimab | 57 (25.79) |
| Toripalimab | 26 (11.76) |
| Camrelizumab | 28 (12.67) |
| Durvalumab | 35 (15.84) |
| Atezolizumab | 40 (18.10) |
| Brain metastases | |
| Yes | 82 (37.10) |
| No | 139 (62.90) |
| Liver metastases | |
| Yes | 57 (25.79) |
| No | 164 (74.21) |
| Bone metastases | |
| Yes | 64 (28.96) |
| No | 157 (71.04) |
No., number; ECOG, Eastern Cooperative Oncology Group; ICIs, immune-checkpoint inhibitors; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Comparison between PD-1 and PD-L1 inhibitors
The baseline characteristics were well balanced according to the Table 2. No significant differences were observed between patients who received PD-1 inhibitors or PD-L1 inhibitors in OS [HR, 1.472; 95% CI, 0.847–2.220; P=0.198; Figure 2A; mOS 19.07 vs. undefined, respectively] and PFS (HR, 0.816; 95% CI, 0.577–1.155; P=0.251; Figure 2A; mPFS 8.27 vs. 7.95, respectively). As showed in the Table 3, the rates of patients showed AEs of any grade treated with PD-1 or PD-L1 were 67.12% and 64.00%, with no significant difference (P=0.642, χ2=0.216), ≥3 grade AEs occurred in 42 (28.76%) and 16 (21.33%) patients treated with PD-1 and PD-L1 inhibitors separately, also no significant difference (P=0.234, χ2=1.415) was observed.
Table 2
| Baseline characteristics | PD-1 | PD-L1 | P value |
|---|---|---|---|
| Gender | 0.113 | ||
| Male | 109 (63.4) | 63 (36.6) | |
| Female | 37 (75.5) | 12 (24.5) | |
| Age, years | 0.451 | ||
| <62 | 74 (68.5) | 34 (31.5) | |
| ≥62 | 72 (63.7) | 41 (36.3) | |
| ECOG | 0.727 | ||
| 0 | 60 (67.4) | 29 (32.6) | |
| 1 | 86 (65.2) | 46 (34.8) | |
| Line of therapy | 0.129 | ||
| First | 66 (61.1) | 42 (38.9) | |
| Second or later | 80 (70.8) | 33 (29.2) | |
| Brain metastases | 0.156 | ||
| Yes | 59 (72.0) | 23 (28.0) | |
| No | 87 (62.6) | 52 (37.4) | |
| Liver metastases | 0.066 | ||
| Yes | 32 (56.1) | 25 (43.9) | |
| No | 114 (69.5) | 50 (30.5) | |
| Osseous metastases | 0.688 | ||
| Yes | 41 (64.1) | 23 (35.9) | |
| No | 105 (66.9) | 52 (33.1) |
Data were presented as n (%). PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; ECOG, Eastern Cooperative Oncology Group.
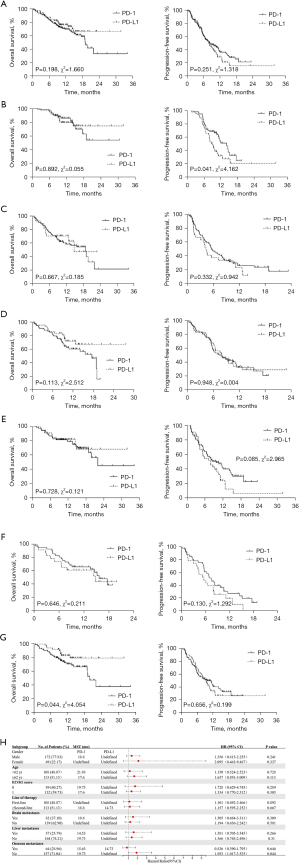
Table 3
| Event | Patients who experienced treatment-related adverse events, No. (%) | ||||
|---|---|---|---|---|---|
| Any grade | Grade ≥3 | ||||
| PD-1 (n=146) | PD-L1 (n=75) | PD-1 (n=146) | PD-L1 (n=75) | ||
| Any treatment-related adverse event | 98 (67.12) | 48 (64.00) | 42 (28.77) | 16 (21.33) | |
| Neutrophil count decreased | 57 (39.04) | 34 (45.33) | 30 (20.55) | 7 (9.33) | |
| White blood cell count decreased | 64 (43.84) | 35 (46.67) | 32 (21.92) | 8 (10.67) | |
| Platelet count decreased | 25 (17.12) | 11 (14.67) | 7 (4.79) | 0 (0) | |
| Anemia | 23 (15.75) | 5 (6.67) | 1 (0.68) | 2 (2.67) | |
| Nausea | 36 (24.66) | 17 (22.67) | 5 (3.42) | 1 (1.33) | |
| Vomiting | 28 (19.18) | 17 (22.67) | 7 (4.79) | 1 (1.33) | |
| Pneumonia | 5 (3.42) | 6 (8.00) | 3 (2.05) | 5 (6.67) | |
| Alanine/aspartate aminotransferase increased | 10 (6.85) | 2 (2.67) | 7 (4.79) | 1 (1.33) | |
PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
A total of 47 patients were treated with PD-1 inhibitors and 61 with PD-L1 inhibitors as the first-line treatment, and no significant differences in OS were observed (HR, 1.101; 95% CI, 0.492–2.466; P=0.892; Figure 2B; the mOS has not been reached), while a longer PFS was observed in patients treated with PD-1 (HR, 0.586; 95% CI, 0.351–0.979; P=0.041; Figure 2B; mPFS 14.13 vs. 8.97, respectively). In second-line or later treatment, patients who received PD-1 or PD-L1 inhibitors achieved comparable OS (HR, 1.157; 95% CI, 0.595–2.252; P=0.667; Figure 2C) and PFS (HR, 0.773; 95% CI, 0.460–1.300; P=0.332; Figure 2C). As shown in Figure 2D and Figure 2E, subgroup analysis of age revealed patients with younger or older age received similar benefits from PD-1 or PD-L1 inhibitors. In patients with bone metastases, no survival differences were observed between PD-1 and PD-L1 treatment (Figure 2F). However, patients without bone metastases were observed to have obtained a better OS treated with PD-L1 inhibitors (HR, 1.893; 95% CI, 1.017–3.525; P=0.044; Figure 2G; mOS 19.73 vs. undefined, respectively). Based on the results of subgroup analysis (Figure 2H), patients without pretreatment bone metastases might have benefited from PD-L1 inhibitors. Patients tended to achieve a better OS when treated with PD-L1 inhibitors, except for those with pretreatment bone metastases.
Comparison of immune check-point inhibitors
We excluded the 2 patients treated with pembrolizumab in consideration of the potential bias. The survival curves of the other 7 ICIs are showed in Figure 3A. There were no significant differences in OS among the different groups (P=0.162, χ2=9.201; Figure 3A) and PFS (P=0.125, χ2=9.988; Figure 3A). Camrelizumab might have achieved a better PFS according to the curves and accordingly, we undertook further studies of camrelizumab vs. other ICIs.
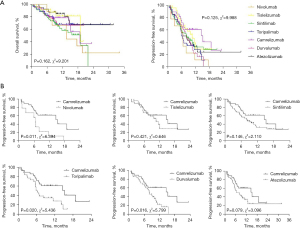
Camrelizumab revealed a longer mPFS (15.17 months) compared with other ICIs (6.47 months for nivolumab, 10.57 months for tislelizumab, 8.10 months for sintilimab, 6.07 months for toripalimab, 7.97 months for durvalumab, and 7.23 months for atezolizumab). Significant differences were only observed in the comparison of nivolumab (HR, 0.256; 95% CI, 0.089–0.736; P=0.011; Figure 3B), toripalimab (HR, 0.434; 95% CI, 0.215–0.875; P=0.020; Figure 3B), and durvalumab (HR, 0.460; 95% CI, 0.245–0.865; P=0.016; Figure 3B).
ICIs in patients with brain metastases
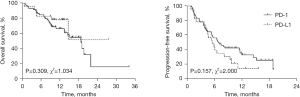
There were 82 patients with asymptomatic brain metastases in our study. The mOS and mPFS of patients with brain metastasis treated with PD-1 were 18.80 and 6.83 months, respectively. The mOS of PD-L1 was undefined, and mPFS was 5.57 months. Neither OS (HR, 1.505; 95% CI, 0.684–3.311; P=0.309; Figure 4) nor PFS (HR, 0.649; 95% CI, 0.356–1.182; P=0.157; Figure 4) showed significant differences.
Comparison of therapies
As of the final follow-up, 7 of the 20 patients treated with ICIs + anti-VEGF/VEGFR agent and 56 of the 173 patients treated with ICIs + chemotherapy have died. The mOS of the ICIs plus anti-VEGF/VEGFR agent group has not been reached, and mPFS was 8.10 months. The mOS of patients who received ICIs plus chemotherapy was 19.73 months, and mPFS was 8.28 months. No significant differences were observed between the two groups in OS (HR, 1.356; 95% CI, 0.563–3.266; P=0.498; Figure 5A) and PFS (HR, 0.829; 95% CI, 0.468–1.470; P=0.521; Figure 5A).
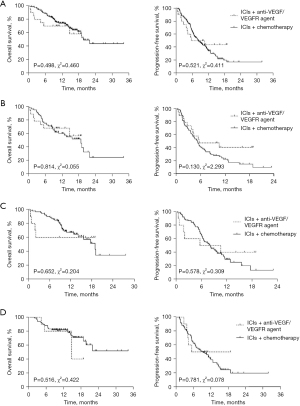
Subgroup analysis was subsequently conducted in consideration of the parameters that might have been related with survival. No significant differences of OS were observed in patients who received second-line or later therapy. However, PFS showed a prolonged tendency in patients treated with ICIs plus anti-VEGF/VEGFR agent as the second-line or later treatment, while patients at different ages seemed to benefit similarly from the two therapies. The subgroup analysis between ICIs + anti-VEGF/VEGFR agent group and ICIs + chemotherapy group in patients received ICIs as second-line or later therapy, with age ≥ 62 years, or with age < 62 years were showed in the Figure 5B-5D.
Discussion
The results of the CASPIAN, IMpower133, and ASTRUM-005 studies (3-4,8) established ICIs combined with chemotherapy as the standard first-line regimen for ES-SCLC patients. Atezolizumab combined with chemotherapy in the IMpower133 study obtained a significant improvement in terms of OS (12.3 vs. 10.3 months; HR, 0.70; 95% CI, 0.54–0.91) and PFS (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62–0.96) (3). The CASPIAN study also demonstrated that durvalumab plus EP significantly improved OS (13.0 vs. 10.3 months; HR 0.73; 95% CI, 0.59–0.91) (4). According to the CAPSTONE-1 study, mOS was significantly longer in the adebrelimab group (15.3 months; 95% CI, 13.2–17.5) compared with the placebo group (12.8 months; HR 0.72; 95% CI, 0.58–0.90) (5). In general, the addition of PD-L1 inhibitors prolonged OS for about 2 months. A 4.5-month prolongation was achieved in the serplulimab group (15.4 months; 95% CI, 13.3 months to not evaluable) compared with the placebo group (10.9 months; 95% CI, 10.0–14.3 months), which demonstrated the importance of PD-1 inhibitors in ES-SCLC treatment (8). The data of the KEYNOTE-604 study showed an mOS of 10.8 months (95% CI, 9.2–12.9 months) in the pembrolizumab plus EP group and 9.7 months (95% CI, 8.6–10.7 months) in the placebo plus EP group. A prolonged OS was also achieved in the pembrolizumab plus EP group (HR, 0.80; 95% CI, 0.64–0.98, P=0.0164; significance threshold P=0.0128), while the significance boundary of OS was not reached (6). The EA5161 study also revealed that nivolumab plus chemotherapy significantly prolonged both PFS (HR, 0.65; 95% CI, 0.46–0.91; mPFS 5.5 vs. 4.6 months) and OS (HR, 0.67; 95% CI, 0.46–0.98; mOS 11.3 vs. 8.5 months) in the intention-to-treat (ITT) population (7). The baseline clinical characteristics of participants in the KEYNOTE-604 study showed that more participants in the pembrolizumab plus EP group had baseline brain metastases (14.5% vs. 9.8%). Prophylactic cranial irradiation (PCI) was given to 27 participants in the pembrolizumab plus EP group (11.8%) and 32 (14.2%) participants in the placebo plus EP group, which might partially explain the failure. The proportion of the participants with brain metastases were smaller in the CASPIAN study, which was 10% in both the durvalumab group and placebo group, 8.5% vs. 8.9% in the Impower133 study, and 2% vs. 2% in the CAPSTONE-1 study. In addition, PD-1 inhibitor could, theoretically, block the binding of PD-1 to both PD-L1 and PD-L2 (9). On the basis of these considerations, the effectiveness of PD-1 inhibitors in the treatment of ES-SCLC patients should be further explored.
Given that a prospective head-to-head comparison between PD-L1 and PD-1 was highly unlikely, further information was obtained from retrospective clinical studies and meta-analyses. According to the meta-analysis of Zhou et al., the addition of PD-1/PD-L1 inhibitors to chemotherapy as the first-line treatment resulted in significant improvements in both PFS and OS for ES-SCLC patients, without an increase in adverse events (AEs). Further, the data demonstrated no significant differences between PD-1 and PD- L1 inhibitors in terms of overall response rate (ORR), PFS and OS (10). Another meta-analysis of PD‑L1 inhibitors vs. PD‑1 inhibitors in firstline treatment with chemotherapy for ES-SCLC revealed that PD-L1 was comparable with PD-1 in terms of OS (HR, 0.99; 95% CI, 0.77–1.23), PFS (HR, 1.10; 95% CI, 0.88–1.37), and ORR [risk ratio (RR), 0.95; 95% CI, 0.81–1.11]. However, PD-L1 inhibitors exhibited a better safety profile in reducing the risk of treatment discontinuation due to AEs and pneumonia (11). Consistent with the meta-analyses mentioned above, we observed no significant differences in OS or PFS between ES-SCLC patients who received PD-1 or PD-L1 inhibitors as second-line or later treatment. However, as the first-line treatment, a longer PFS was observed in patients treated with PD-1 (HR, 0.586; 95% CI, 0.351–0.979; P=0.041). According to another meta-analysis that compared PD-1 and PD-L1 inhibitors in patients with solid tumors, a pooled greater OS benefit of PD-1 inhibitors was observed (HR, 0.75; 95% CI, 0.65–0.86; P<0.001), as well as for PFS (HR, 0.73; 95% CI, 0.56–0.96; P=0.020) (12). And according to our data, the rate of AEs occurred in PD-1and PD-L1 treatment were similar no matter of and grade or ≥3 grade. All the evidence suggested that PD-1 inhibitors might perform comparably with PD-L1 inhibitors in ES-SCLC treatment. But the evidence was not sufficient enough because of the small number of enrolled cases and the confounding factors existed.
In the KEYNOTE-604 study, the HR favored placebo plus EP in the subgroup with brain metastases (HR, 1.32; 95% CI, 0.72–2.42), which was the same as the IMpower133 study (HR, 1.07; 95% CI, 0.47–2.43), while CASPIAN, ASTRUM-005, and CAPSTONE-1 revealed better results with ICIs plus chemotherapy in patients with brain metastases. According to Yu et al. (11), there was a tendency for reduced risk of death in patients with brain metastases receiving PD-L1 inhibitors compared with PD-1 inhibitors (HR, 0.61; 95% CI, 0.28–1.32). We observed the same tendency in subgroup analysis, with the HR favoring PD-L1 inhibitors (HR, 1.28; 95% CI, 0.55–2.99). However, further investigation showed that there were no significant differences in OS and PFS between PD-1 and PD-L1 inhibitors.
VEGF plays a crucial role in promoting the abnormal tumor vasculature and also acts as an immunosuppressant (13,14). The results of previous animal experiments showed that anti-VEGF/VEGFR agent improved the immunosuppressive environment, providing rationale for the combination of immunotherapy and anti-VEGF/VEGFR agent (15). The combination of camrelizumab and apatinib in the second-line treatment of ES-SCLC patients revealed an ORR of 34.0%, an mOS of 8.4 months (95% CI, 4.7‒12.3), and mPFS of 3.6 months (95% CI, 1.9‒4.6) (16), which were comparable to the present standard second-line treatment with topotecan (mOS, 6.25–8.75 months; mPFS, 3.33–3.65 months) (17-20) and amrubicin (ORR 31.1%, mOS 7.5 months, and mPFS 4.1 months) (19). Therefore, we compared treatment efficacy between ES-SCLC patients who received ICIs combined with anti-VEGF/VEGFR agent or chemotherapy, and the results showed that there was no significant difference between the two groups in OS (HR, 1.356; 95% CI, 0.563–3.266; P=0.498) and PFS (HR, 0.829; 95% CI, 0.468–1.470; P=0.521). These results indicated that the synergetic role of anti-VEGF/VEGFR agent in immunotherapy should receive further attention.
As a retrospective study, the small number of enrolled cases of patients treated with PD-L1 inhibitors, recall bias, loss of follow-up bias, and data heterogeneity, might have affected the accuracy of the results to some extent. However, as a real-world study, our research had the advantages of including broader populations, increasing efficiency, and reflecting the actual use of drugs in practice (21).
Conclusions
Our results suggested that PD-1 and PD-L1 inhibitors might provide a comparable survival benefit in second-line or later treatment, while a longer PFS was observed in ES-SCLC patients treated with PD-1 inhibitors as the first-line treatment. No significant differences were observed in ES-SCLC patients with brain metastases treated with PD-1 or PD-L1 inhibitors. In addition, a comparison between different ICIs revealed no significant differences in term of OS and PFS, except that camrelizumab seemed to achieve a better PFS. Further, ICIs combined with chemotherapy or anti-VEGF/VEGFR agent revealed similar efficacy.
Acknowledgments
The abstract was presented in part at the ASTRO’s 64th Annual Meeting; October 23-26, 2022; San Antonio.
Funding: This study was funded by grants from the Key Research and Development Program of Shandong: Major Science & Technology Innovation Project (No. 2021SFG0501) and Research and clinical application of key technologies of precision radiotherapy combined with immunotargeted therapy for esophageal cancer (No. 2021LCZX04).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1682/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1682/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1682/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shandong Cancer Hospital (No. SDTHEC 2022011009). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Frese KK, Simpson KL, Dive C. Small cell lung cancer enters the era of precision medicine. Cancer Cell 2021;39:297-9. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Goldman JW, Garassino MC, Chen Y, et al. Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study. Lung Cancer 2020;149:46-52. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369-79. [Crossref] [PubMed]
- Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol 2020;38:9000.
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv Exp Med Biol 2020;1248:33-59. [Crossref] [PubMed]
- Zhou F, Zhao W, Gong X, et al. Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer. J Immunother Cancer 2020;8:e001300. [Crossref] [PubMed]
- Yu H, Chen P, Cai X, et al. Efficacy and safety of PD-L1 inhibitors versus PD-1 inhibitors in first-line treatment with chemotherapy for extensive-stage small-cell lung cancer. Cancer Immunol Immunother 2022;71:637-44. [Crossref] [PubMed]
- Duan J, Cui L, Zhao X, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6:375-84. [Crossref] [PubMed]
- Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 2013;73:2943-8. [Crossref] [PubMed]
- Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol 2018;9:978. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Fan Y, Zhao J, Wang Q, et al. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial. J Thorac Oncol 2021;16:299-309. [Crossref] [PubMed]
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086-92. [Crossref] [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [Crossref] [PubMed]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [Crossref] [PubMed]
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med 2016;375:2293-7. [Crossref] [PubMed]
(English Language Editor: A. Muylwyk)


