
The association of postoperative radiotherapy with survival in resected N2 non-small cell lung cancer
Introduction
Lung cancer is responsible for 18.4% of all cancer deaths, making it the leading cause of cancer-associated mortality worldwide (1). Patients with resected pathologic N2 non-small cell lung cancer (NSCLC) are a high-risk group for regional recurrence and metastasis, even with complete resection (2). The National Comprehensive Cancer Network (NCCN) guidelines currently recommend adjuvant chemotherapy for patients who undergo resection for pathologic N2 NSCLC (3); however, there a lack of consensus regarding the benefit of postoperative radiotherapy (PORT) for this group.
Data from two large databases, Surveillance, Epidemiology, and End Results (SEER) (4) and National Cancer Database (NCDB) (5), suggest that PORT can improve survival for patients with resected pathologic N2 NSCLC. Meta-analyses have also revealed a benefit of PORT in N2 nodal disease (6,7). Further, the subgroup investigation of the Lung ART trial verified that PORT could decrease the local recurrence rate for these patients. However, other studies have suggested that PORT has no significant effect on survival (8-12), and the NCCN guidelines do not give a clear recommendation on whether PORT is required (3). Therefore, the role of PORT in the treatment of completely resected N2 NSCLC is still highly controversial.
In addition to the lack of evidence to suggest that PORT can be beneficial to patients, the current staging system for completely resected pathologic N2 NSCLC treated with chemotherapy is not sufficient for identifying those patients who are most likely to benefit from PORT. Therefore, developing a survival model exploring the potential individual benefit of PORT remains necessary.
In this study, we aimed to develop a survival prediction model to calculate the probable overall survival (OS) differences with or without PORT in patients with completely resected pathologic N2 NSCLC treated with chemotherapy. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-772/rc).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University approved this study (approval No. 2020 No. 69; issue date: 13/3/2020). Individual consent for this retrospective analysis was waived.
Information of patients with completely resected pathologic N2 NSCLC treated with chemotherapy from between 2002 and 2014 was extracted from the SEER database (http://seer.cancer.gov/). Patients were included if they: had pathologically confirmed primary N2 NSCLC between January 2002 and December 2014, had a history of complete resection through lobectomy or pneumonectomy, had received treatment with chemotherapy, and had only one malignant primary lesion. Patients were excluded if they: had distant metastasis; had invasion of the heart, great vessels, trachea, diaphragm, mediastinum, recurrent laryngeal nerve, carina, vertebral body, or esophagus; had undergone preoperative radiotherapy; had radioactive implants; had received radioisotopes; or had information missing from their extracted data.
An external validation cohort that met the same inclusion and exclusion criteria was included to analyze the applicability of the prediction model. The cohort consisted of 602 patients treated between 2009 and 2014 in the First Affiliated Hospital of Guangzhou Medical University and the Collaborative Innovation Center for Cancer Medicine of Sun Yat-sen University, China.
Baseline data of the demographics of the patients (age, sex, and race), tumor characteristics (size, location, differentiation grade, and histological type), the number of examined lymph nodes, the number of positive lymph nodes, the extent of surgery, and visceral pleural invasion (VPI) were gathered from the SEER database. The TNM categories were based on the International Association for the Study of Lung Cancer (IASLC) eighth edition staging system (13). Cases were categorized as having received or not received PORT (the PORT group and non-PORT group, respectively).
Construction of the nomogram
In the training set for the PORT and non-PORT groups, OS was predicted with the Kaplan–Meier method and analyzed by applying the log-rank test. Multivariable Cox proportional hazards regression was applied to identify independent prognostic factors. On the basis of the significant independent factors in the two groups, nomograms were formulated using R version 3.5.3 (R Core Team, Vienna, Austria) with the rms and survival packages (14). The rms package corresponds with the book Regression Modeling Strategies. All survival models were constructed using the rms R library by Harrell (http://cran.r-project.org/web/packages/rms).
Validation and calibration of the nomogram
The model was subjected to 1,000 bootstrap resamples for internal validation in the training cohort and external validation in the cohort from the Chinese Institute. Calibration for 1-, 3-, and 5-year OS was determined by comparing the predicted survival with that observed on 1,000 bootstrap resamples. The discrimination ability of the model was determined using the concordance index (C-index). C-index values range from 0.5 to 1.0, with a higher value suggesting a better predictive performance (15). The C-index values for the two different models were compared using methods previously described (16).
Statistical analysis
The chi-square test was applied to examine the statistical significance of the differences in clinical variables between the PORT and non-PORT groups. OS was calculated using the Kaplan–Meier method and compared by applying the log-rank test. Independent prognostic factors were identified using multivariate Cox proportional hazards regression, and the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were determined. The nomograms were developed using the rms package of R version 3.5.3 (R Core Team, Vienna, Austria). All statistical analyses were performed using SPSS 22.0 (IBM Corp, Armonk, NY, USA), and a P value less than 0.05 was regarded as statistically significant.
Results
Clinical characteristics
A total of 3,094 patients with completely resected pathologic N2 NSCLC who were treated with chemotherapy derived from the SEER database (Figure S1), and 602 patients from a multicenter hospital in China, met the inclusion criteria. The demographics and clinicopathological characteristics of patients in the training and external validation cohorts are listed in Table 1. The median interquartile range and follow-up times on OS were 27 months [13, 52] and 36 months [22, 49] in the training cohort and external validation cohort, respectively.
Table 1
| Characteristic | Training cohort | External validation cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PORT (n=1,519) | No PORT (n=1,575) | P | PORT (n=69) | No PORT (n=533) | P | ||||||||
| No. | % | No. | % | No. | % | No. | % | ||||||
| Age, years | 0.001 | 0.127 | |||||||||||
| <60 | 517 | 34 | 495 | 31.4 | 47 | 68.1 | 295 | 55.3 | |||||
| 60–70 | 594 | 39.1 | 558 | 35.4 | 17 | 24.6 | 177 | 33.2 | |||||
| ≥70 | 408 | 26.9 | 522 | 33.1 | 5 | 7.2 | 61 | 11.4 | |||||
| Sex | 0.272 | 0.25 | |||||||||||
| Male | 734 | 48.3 | 730 | 46.3 | 45 | 65.2 | 309 | 58 | |||||
| Female | 785 | 51.7 | 845 | 53.7 | 24 | 34.8 | 224 | 42 | |||||
| Race | 0.674 | ||||||||||||
| White | 1,236 | 81.4 | 1,285 | 81.6 | – | – | – | – | |||||
| Black | 138 | 9.1 | 152 | 9.7 | – | – | – | – | |||||
| Other | 145 | 9.5 | 138 | 8.8 | – | – | – | – | |||||
| Location | 0.732 | 0.856 | |||||||||||
| Upper | 875 | 57.6 | 896 | 56.9 | 31 | 44.9 | 258 | 48.4 | |||||
| Middle | 75 | 4.9 | 72 | 4.6 | 7 | 10.1 | 41 | 7.7 | |||||
| Lower | 516 | 34 | 548 | 34.8 | 28 | 40.6 | 206 | 38.6 | |||||
| Other | 53 | 3.3 | 59 | 3.7 | 3 | 4.3 | 28 | 5.3 | |||||
| Examined lymph nodes | 0.035 | 0.837 | |||||||||||
| 0–9 | 696 | 45.8 | 649 | 41.2 | 7 | 10.1 | 46 | 8.6 | |||||
| 10–15 | 404 | 26.6 | 453 | 28.8 | 13 | 18.8 | 114 | 21.4 | |||||
| ≥16 | 419 | 27.6 | 473 | 30 | 49 | 71 | 373 | 70 | |||||
| Positive lymph nodes | 0.007 | 0.963 | |||||||||||
| 1–3 | 876 | 57.7 | 995 | 63.2 | 26 | 37.7 | 210 | 39.4 | |||||
| 4–9 | 531 | 35 | 478 | 30.3 | 28 | 40.6 | 211 | 39.6 | |||||
| ≥10 | 112 | 7.4 | 102 | 6.5 | 15 | 21.7 | 112 | 21 | |||||
| Tumor size, cm | |||||||||||||
| ≤3 | 715 | 47.1 | 719 | 45.7 | 0.305 | 30 | 43.5 | 259 | 48.6 | 0.524 | |||
| >3 to 5 | 522 | 34.4 | 525 | 33.3 | 29 | 42 | 183 | 34.3 | |||||
| >5 to 7 | 187 | 12.3 | 230 | 14.6 | 5 | 7.2 | 58 | 10.9 | |||||
| >7 | 95 | 6.3 | 101 | 6.4 | 5 | 7.2 | 33 | 6.2 | |||||
| Extent of surgery | 0.01 | 0.3 | |||||||||||
| Lobectomy | 1,385· | 91.2 | 1,392 | 88.4 | 68 | 98.6 | 512 | 96.1 | |||||
| Pneumonectomy | 134 | 8.8 | 183 | 11.6 | 1 | 1.4 | 21 | 3.9 | |||||
| Differentiation grade | 0.834 | 0.126 | |||||||||||
| Grade I | 72 | 4.7 | 83 | 5.3 | 1 | 1.4 | 17 | 3.2 | |||||
| Grade II | 633 | 41.7 | 663 | 42.1 | 33 | 47.8 | 289 | 54.2 | |||||
| Grade III or IV | 713 | 46.9 | 733 | 46.5 | 19 | 27.5 | 158 | 29.6 | |||||
| Unknown | 101 | 6.6 | 96 | 6.1 | 16 | 23.2 | 69 | 12.9 | |||||
| Histology | 0.71 | 0.007 | |||||||||||
| SC | 273 | 18 | 305 | 19.4 | 23 | 33.3 | 93 | 17.4 | |||||
| Adenocarcinoma | 1,028 | 67.7 | 1,038 | 65.9 | 42 | 60.9 | 405 | 76 | |||||
| Others | 218 | 14.4 | 232 | 14.7 | 4 | 5.8 | 35 | 6.6 | |||||
| VPI | 0.195 | 0.968 | |||||||||||
| Yes | 537 | 35.4 | 522 | 33.1 | 34 | 49.3 | 264 | 49.5 | |||||
| No | 982 | 64.6 | 1,053 | 66.9 | 35 | 50.7 | 269 | 50.5 | |||||
PORT, postoperative radiotherapy; SC, squamous carcinoma; VPI, visceral pleural invasion.
Independent prognostic factors in the training cohort
Survival analysis using the log-rank test found no significant differences in OS (HR =1.006; 95% CI: 0.915–1.106; P=0.9) between the PORT and non-PORT groups (Figure 1). Results from the multivariate regression model are listed in Table 2. For patients with PORT, the multivariate analysis indicated that age (P<0.001), sex (P=0.011), number of examined lymph nodes (P<0.001), number of positive lymph nodes (P<0.001), tumor size (P=0.037), extent of surgery (P=0.032), and differentiation grade (P=0.001) were independent prognostic factors for OS. For patients in the non-PORT group, the multivariate analysis indicated that age (P<0.001), sex (P<0.001), examined lymph nodes (P=0.013), the number of positive lymph nodes (P<0.001), tumor size (P=0.005), and VPI (P=0.046) were independent prognostic factors for OS.

Table 2
| Characteristic | PORT (n=1,519) | No PORT (n=1,575) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis, P value | Multivariable analysis | Univariable analysis, P value | Multivariable analysis | ||||||
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||||
| Age, years | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| <60 | 1 (reference) | 1 (reference) | |||||||
| 60–70 | 1.203 | 1.019 to 1.419 | 0.029 | 1.100 | 0.927 to 1.306 | 0.274 | |||
| ≥70 | 1.531 | 1.283 to1.828 | <0.001 | 1.642 | 1.387 to 1.944 | <0.001 | |||
| Sex | 0.003 | <0.001 | |||||||
| Male | 1 (reference) | 1 (reference) | |||||||
| Female | 0.835 | 0.727 to 0.959 | 0.011 | 0.702 | 0.613 to 0.803 | <0.001 | |||
| Race | 0.238 | 0.011 | 0.107 | ||||||
| White | – | – | – | 1 (reference) | |||||
| Black | – | – | – | 0.983 | 0.779 to 1.242 | 0.981 | |||
| Other | – | – | – | 0.759 | 0.587 to 0.980 | 0.034 | |||
| Location | 0.046 | 0.301 | 0.069 | 0.814 | |||||
| Upper | 1 (reference) | 1 (reference) | |||||||
| Middle | 0.982 | 0.694 to 1.388 | 0.916 | 1.040 | 0.74 to 1.461 | 0.82 | |||
| Lower | 1.034 | 0.889 to 1.203 | 0.663 | 1.073 | 0.928 to 1.242 | 0.34 | |||
| Other | 0.650 | 0.313 to 1.350 | 0.248 | 0.995 | 0.692 to 1.432 | 0.98 | |||
| Examined lymph nodes | 0.075 | <0.001 | 0.435 | 0.013 | |||||
| 0–9 | 1 (reference) | 1 (reference) | |||||||
| 10–15 | 0.792 | 0.666 to 0.942 | 0.008 | 0.842 | 0.714 to 0.993 | 0.041 | |||
| ≥16 | 0.660 | 0.546 to 0.797 | <0.001 | 0.775 | 0.649 to 0.927 | 0.005 | |||
| Positive lymph nodes | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| 1–3 | 1 (reference) | 1 (reference) | |||||||
| 4–9 | 1.423 | 1.218 to 1.662 | <0.001 | 1.345 | 1.155 to 1.568 | <0.001 | |||
| ≥10 | 1.849 | 1.397 to 2.448 | <0.001 | 1.841 | 1.400 to 2.423 | <0.001 | |||
| Tumor size, cm | <0.001 | 0.037 | <0.001 | 0.005 | |||||
| ≤3 | 1 (reference) | 1 (reference) | |||||||
| >3 to 5 | 1.145 | 0.978 to 1.340 | 0.92 | 1.150 | 0.984 to 1.345 | 0.08 | |||
| >5 to 7 | 1.341 | 1.083 to 1.660 | 0.007 | 1.241 | 1.007 to 1.528 | 0.042 | |||
| ≥7 | 1.245 | 0.934 to 1.661 | 0.135 | 1.605 | 1.221 to 2.109 | 0.001 | |||
| Extent of surgery | 0.007 | 0.016 | |||||||
| Lobectomy | 1 (reference) | 1 (reference) | |||||||
| Pneumonectomy | 1.308 | 1.024 to 1.671 | 0.032 | 1.117 | 0.896 to 1.391 | 0.326 | |||
| Differentiation grade | 0.011 | 0.001 | 0.142 | 0.317 | |||||
| Grade I | 1 (reference) | 1 (reference) | |||||||
| Grade II | 1.338 | 0.895 to 2.000 | 0.156 | 1.254 | 0.906 to 1.735 | 0.173 | |||
| Grade III or IV | 1.721 | 1.154 to 2.565 | 0.008 | 1.335 | 0.966 to 1.846 | 0.08 | |||
| Unknown | 1.639 | 1.010 to 2.660 | 0.045 | 1.365 | 0.903 to 2.063 | 0.14 | |||
| Histology | 0.374 | – | – | – | 0.825 | – | – | – | |
| SC | – | – | – | – | – | – | |||
| Adenocarcinoma | – | – | – | – | – | – | |||
| Others | – | – | – | – | – | – | |||
| VPI | 0.029 | 0.012 | |||||||
| Yes | 1 (reference) | 1 (reference) | |||||||
| No | 1.146 | 0.991 to 1.326 | 0.66 | 1.157 | 1.003 to 1.335 | 0.046 | |||
PORT, postoperative radiotherapy; CI, confidence interval; SC, squamous carcinoma; VPI, visceral pleural invasion.
Development of the prognostic nomogram
Nomograms were constructed from the coefficients from the multivariate regression model. Significant independent factors in the two groups, including age, sex, the number of examined lymph nodes, the number of positive lymph nodes, tumor size, the extent of surgery, differentiation grade, and VPI, were included to develop the nomograms. The first nomogram (Figure 2A) estimated OS with PORT, and the second nomogram (Figure 2B) estimated OS without PORT.
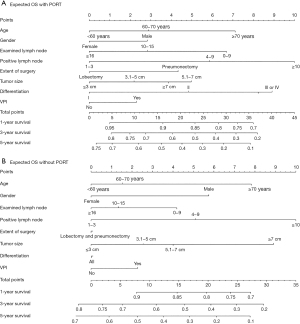
To estimate the net survival benefit of PORT, the two nomograms were used together (Figure 2). The difference between the two estimates represented the expected net survival benefit from the addition of PORT. Each factor was given a score on the point scale. By calculating the total score, finding it on the total point scale, and drawing a straight line, the estimated probability of survival at each score point could be easily determined.
Calibration and validation of the nomogram
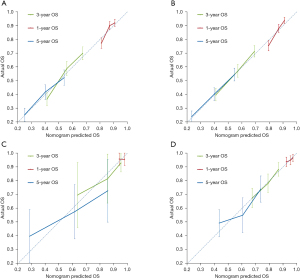
In the training cohort, the calibration curves (Figure 3A,3B) showed strong agreement between the 1-, 3-, and 5-year OS predicted by the nomogram and that actually observed. In the PORT group, the value of Harrell’s C-index for the nomogram established to predict OS (0.619; 95% CI: 0.598–0.641) was significantly greater than that of the IASLC eighth edition staging system (T1, T2, T3, and T4, 0.566; 95% CI: 0.521–0.610; P<0.01). In the non-PORT group, the C-index was higher for the nomogram 0.627 (95% CI: 0.605–0.648) than it was for the T category prediction (0.559; 95% CI: 0.540–0.610; P<0.01). In the external validation cohort, the calibration plots also presented acceptable agreement between the nomogram predictions and actual observations for 1-, 3-, and 5-year OS (Figure 3C,3D). The C-index was 0.599 (95% CI: 0.485–0.713) for the PORT group and 0.595 (95% CI: 0.544–0.646) for the non-PORT group.
Clinical use
For each individual patient, we used nomogram A to first calculate the expected OS with PORT, then we used nomogram B to calculate the expected OS without PORT. The difference between the two estimates represented the expected net survival difference from the addition of PORT. The net survival difference was computed for each patient based on their 3-year survival rates calculated using the two nomograms.
In the SEER dataset, there were 1,434 patients with a positive PORT net survival difference and 1,475 patients with a negative PORT net survival difference. The multivariate analyses showed that PORT could improve OS (HR: 0.861; 95% CI: 0.744–0.996; P=0.044) for patients with a positive PORT net survival difference. However, for patients with a negative PORT net survival difference, PORT was not significantly associated with OS (HR: 1.113; 95% CI: 0.978–1.267; P=0.105). The Kaplan–Meier curves for OS are shown in Figure S2.
Discussion
We established a practical survival prediction model that can be used to make individualized predictions about the expected survival benefit of PORT for patients with completely resected pathologic N2 NSCLC treated with chemotherapy. For the patients in our analysis, the model had a better predictive performance than the T category of the IASLC eighth edition staging manual, as indicated by its higher C-index. Our model is practical for individualized recommendations for the use of PORT.
Adjuvant chemotherapy is recommended for patients with completely resected pathologic N2 by the NCCN guidelines (3). Therefore, our study excluded patients who had not undergone chemotherapy. In this large population-based study, our results revealed no statistical differences in OS between the PORT and non-PORT groups (Figure 1). Consistent with this result, an early closed randomized controlled trial indicated that PORT increased both the local/regional and distant disease-free survival rates but not the OS rate (9). A randomized phase III study (8) with 37 patients and a phase II trial with 101 patients (10) also showed that there were no statistical differences in OS between the observation and PORT arms. Further, a randomized controlled trial (Lung-Art Trial, NCT00410683) of the ESMO (European Society for Medical Oncology) congress and the most recent phase III clinical trial (12) results showed that PORT had no progression-free survival PFS or OS benefit for patients with R0 resection N2 (IIIA) of NSCLC. Both studies mentioned prognostic models considering disease-free survival but suggested that further studies were needed to investigate the benefit of PORT for patients. Our study based on a large cohort from the SEER database obtained the same result for OS. However, previous retrospective studies and meta-analyses found that PORT could significantly improve the survival of patients (4-7). Therefore, the benefit of PORT for patients with completely resected N2 is still controversial. Our nomograms may also be valuable for identifying those patients most likely to benefit from PORT; using the nomograms, we found that approximately half of the patients in our study might have benefited from PORT (Figure S2).
It is unclear why the results of our study differ from those of others (4,5). A possible explanation is the difference in the prognostic factors included in the multivariate analyses. For example, the numbers of positive lymph nodes and examined lymph nodes were not reported in previous NCDB studies (5,17). However, the number of positive lymph nodes is an essential prognostic factor in many cancers, and comparable studies have suggested that a higher number of positive lymph nodes (n>3) is associated with a poorer survival rate (18,19). Further, examined lymph node count is also an important prognostic factor for NSCLC (20). The independent prognostic factors of age, sex, tumor size, and extent of surgery in our study were also identified in some prior studies for NSCLC (5,21,22). Our study did not select histology as a candidate factor because it was not an independent prognostic factor. This finding is consistent with those of other studies on stage II or III (4) and IIIA-N2 (22) NSCLC based on SEER data. Further, we found that VPI is associated with poor prognosis in N2 stage NSCLC. The cutoff point of examined lymph nodes are mostly based on our previous studies (20,21). A cutoff point of 3 positive lymph nodes was recommended (18). Therefore, age, sex, number of examined lymph nodes, number of positive lymph nodes, tumor size, extent of surgery, differentiation grade, and VPI were the factors ultimately included in the nomogram.
The calibration plots in the training and external validation cohorts showed ideal agreement between actual OS and the nomogram-predicted OS, indicating that the predictive functionality of the nomograms was excellent. The C-index for our nomograms—0.63 and 0.66 for the PORT and non-PORT cohorts, respectively—were superior to those of TNM staging (0.56 and 0.55, respectively) for OS, with a P value of less than 0.001. Therefore, by combining multiple clinical risk factors, our nomogram have a better discrimination ability than the TNM staging system. Moreover, considering that the data were gathered from the United States’ multicenter SEER database and two centers in China—which might reduce the impact of patient history backgrounds and hospital differences—the nomograms can be generally applied.
Although several NSCLC prognostic models had been reported previously (20-24), no nomogram had been developed for completely resected pathologic N2 NSCLC with and without PORT. We had previously developed a survival model to predict OS for patients with stage I–IIIA resected NSCLC, but only 24% of the cases included were N2 and it was not related to PORT (20). A recent study proposed a nomogram to predict the survival of patients with stage IIIA–N2 NSCLC after surgery (22); however, it lacked chemotherapy data and could not guide the choice of PORT. Jiang et al. (25) reported a similar survival prediction model for patients with stage II or III gastric cancer. Their nomograms can be applied to calculate individualized predictions of the probable OS advantage from adjuvant chemotherapy for these patients. We also established practical nomograms to predict OS and identified a subset of patients who might benefit from PORT.
There are some limitations to our study. First, the clinical characteristics of patients with and without PORT differed slightly between the training and external validation cohorts (Table 1); in particular, only a very low proportion of the Chinese patients had received PORT. Therefore, our results should be further validated using large multicenter data from other countries. Second, our study was limited by its retrospective design, which introduced unavoidable bias. The only way to solve this issue is by carrying out a well-conducted phase III trial. Further, our study lacked data on some relevant molecular factors, the chemotherapy regimen and cycle, tumor recurrence, DFS, radiotherapy details, surgical margin status, and comorbidity. Moreover, the C-indices of the nomogram were only 0.619 and 0.627, which is not inspiring. Future studies using prospective data collection and additional prognostic variables are needed to improve the performance and reliability of the model.
Conclusions
We have established a practical nomogram that can produce an individualized estimate of the net survival difference with or without PORT for patients with completely resected pathologic N2 NSCLC who have received chemotherapy. This model can help to quantify the survival benefit of PORT after surgical resection of N2 NSCLC with chemotherapy and can assist in making individualized therapeutic decisions.
Acknowledgments
Funding: This study was supported by Guangdong Provincial Basic and Applied Basic Research Fund of China (Grant No. 2020A1515110445); the China State Key Laboratory of Respiratory Disease Independent Subject (Grant No. SKLRD-QN-201925); and the National Key R & D Program of China (Grant No. 2017YFC0907903 & 2017YFC0112704).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-772/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-772/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-772/coif). JH serves as Executive Editor-in-Chief of the Journal of Thoracic Disease. WL served as an unpaid editorial board member of the Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [Crossref] [PubMed]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: NonSmall Cell Lung Cancer. V. 1. 2017. Available online: www.nccn.org/professionals/physician_gls/PDF/nscl.pdf
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998-3006.
- Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 2015;33:870-6. [Crossref] [PubMed]
- Sakib N, Li N, Zhu X, et al. Effect of postoperative radiotherapy on outcome in resectable stage IIIA-N2 non-small-cell lung cancer: an updated meta-analysis. Nucl Med Commun 2018;39:51-9. [Crossref] [PubMed]
- Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol 2014;110:3-8. [Crossref] [PubMed]
- Perry MC, Kohman LJ, Bonner JA, et al. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non-small-cell lung cancer: Cancer and Leukemia Group B 9734. Clin Lung Cancer 2007;8:268-72. [Crossref] [PubMed]
- Shen WY, Ji J, Zuo YS, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: an early closed randomized controlled trial. Radiother Oncol 2014;110:120-5. [Crossref] [PubMed]
- Sun JM, Noh JM, Oh D, et al. Randomized Phase II Trial Comparing Chemoradiotherapy with Chemotherapy for Completely Resected Unsuspected N2-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1806-13. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Hui Z, Men Y, Hu C, et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol 2021;7:1178-85. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Frank E HJR. Regression Modeling Strategies. R Package version 3.4-0. Available online: http://www.rproject.org/
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43. [Crossref] [PubMed]
- Wong AT, Rineer J, Schwartz D, et al. Assessing the Impact of Postoperative Radiation Therapy for Completely Resected Limited-Stage Small Cell Lung Cancer Using the National Cancer Database. J Thorac Oncol 2016;11:242-8. [Crossref] [PubMed]
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5.
- Shang X, Liu J, Li Z, et al. A hypothesized TNM staging system based on the number and location of positive lymph nodes may better reflect the prognosis for patients with NSCLC. BMC Cancer 2019;19:591. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Zeng Y, Mayne N, Yang CJ, et al. A Nomogram for Predicting Cancer-Specific Survival of TNM 8th Edition Stage I Non-small-cell Lung Cancer. Ann Surg Oncol 2019;26:2053-62.
- Mao Q, Xia W, Dong G, et al. A nomogram to predict the survival of stage IIIA-N2 non-small cell lung cancer after surgery. J Thorac Cardiovasc Surg 2018;155:1784-92.e3. [Crossref] [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [Crossref] [PubMed]
- Birim O, Kappetein AP, Waleboer M, et al. Long-term survival after non-small cell lung cancer surgery: development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491-8. [Crossref] [PubMed]
- Jiang Y, Li T, Liang X, et al. Association of Adjuvant Chemotherapy With Survival in Patients With Stage II or III Gastric Cancer. JAMA Surg 2017;152:e171087. [Crossref] [PubMed]
(English Language Editors: C. Mullens and J. Reylonds)


