Translocation of left inferior lobe pulmonary artery to the pulmonary artery trunk for central type non-small cell lung cancers
Introduction
Recent studies have demonstrated that sleeve lobectomy is superior in functional performance, morbidity, and quality-of-life outcomes compared to pneumonectomy, and it provides an oncologic outcome equivalent to a pneumonectomy (1-3).
Although a pulmonary artery (PA) sleeve or plasty combined with bronchial sleeve resection for lobectomy is a routine practice in most thoracic surgical centers, translocation of the left inferior lobar PA to the PA trunk associated with bronchial sleeve resection of the left superior lobe has been rarely reported. Herein, we report on four patients with non-small-cell lung cancer (NSCLC) who underwent this operation. A literature review is also included with a summary of surgical indications, techniques, management, perioperative complications, and survival.
Methods
Approval for this study was obtained from the Ethics Committee of Shanghai Chest Hospital. Patients or significant others signed informed consent statements for operation, data collection, and follow-up telephone contact or interviews.
Patients
Translocation of the left inferior lobe PA to the PA trunk and sleeve resection for the left superior lobe for treatment of central-type non-small cell lung cancers was performed in our department between June 13, 2014 and June 8, 2015. Four patients were male, with a median age of 60.5 (range, 52–67) years. The symptoms on presentation included persistent dry cough and bloody sputum.
Pre-operative evaluation and treatment
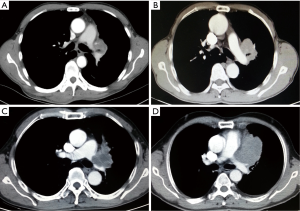
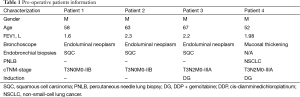
On chest CT examinations, all tumors were located in the upper left lobe, and they ranged in the diameter from 4.5 to 7.5 cm. The initial segment of the left PA and pericardium were invaded by direct tumor extension (Figure 1A-D). The arterial ligaments were invaded, and they even disappeared. However, the PA bifurcation in the pericardium was excluded from invasion under CT scan. Despite the tumor extension, both the distal segment of the left PA and the lower segment of the bronchus were free of tumor involvement. Further calculation of image reconstruction revealed that the mean tumor extension length in the PA was approximately 4.1±0.85 cm on the PA. Additionally, endo-bronchus information was obtained from bronchoscopies, which showed mucosal thickening and bronchial narrowing in 1 case and endoluminal neoplasm in 3 cases at the orifice of the upper left lobar orifice. We obtained cytology types as squamous cell carcinoma in three cases via bronchoscopies, and we extricated NSCLC cells from a CT guided transthoracic needle biopsy of patient with negative bronchoscopies. All patients underwent brain magnetic resonance imaging (MRI), entire body bone scan, and upper abdominal CT scan to exclude remote metastasis. Considering the locally advanced nature of the tumors in four patients, we suggested neoadjuvant chemotherapy before operation. Two patients refused to undergo this procedure and chose immediate surgical intervention as the first option (No. 1 and No. 2). Two cycles of preoperative chemotherapy with cis-platinum-based regimens were administered in two patients (No. 3 and No. 4). (Pre-operative patient’s information is shown in Table 1). Re-evaluations were performed before operations to exclude contraindications after neoadjuvant chemotherapy.
Full table
Surgical manipulations
The operation was performed through the fourth intercostals of the left posterior lateral incisions in three patients and through the median sternotomies in one patient. The mediastinal structures involved by the tumor and the possibility of reconstruction were first evaluated on exploration. Tumors were surrounded by the tracheobronchial angle, left main PA, superior pulmonary vein, and pericardium. Generally, a pneumonectomy is the choice for this kind of tumor. The PA bifurcation and intrapericardial superior pulmonary vein were not involved by the tumor when opening the pericardium. As the distal segment of the left PA and the lower segment of the bronchus were not infiltrated by the tumor, we translocated the left inferior lobe PA to the intrapericardial PA trunk, and bronchus sleeve resected the left superior lobe.
The first step was to release the superior pulmonary vein and PA bifurcation in the pericardium. The pericardium was opened to the largest extent possible along the edge of the tumor to facilitate encircling of the PA and superior pulmonary vein. Before a complete resection, the vein must be preserved and left unligated.
The second step involved sleeve lobectomy. If a cancerous extension involved the lower segment of PA branch or lower bronchial orifice, further reconstruction would be contraindicated. Opening of the pericardium facilitated an easy exposure of the PA trunk and bifurcation of the PA. Because of the involvement of the left PA beyond the arterial ligament, a Linear Cutter Stapler approach for dissecting and cutting the left PA was used to close the left PA orifice at the position of PA bifurcation.
The third step was en bloc removal of the involved lobe, pericardium, and phrenic nerve, followed by plastic reconstruction bronchus and PA in sequence. The superior Lobe was sleeve resected after extensive dissection of an oblique fissure, the left superior lobar bronchus and lower segment of the left PA. A tumor-free border of the bronchus and the PA was guaranteed through a margin frozen section. The distal PA was clamped using bulldog vessel clamps after the administration of 5,000 IU heparin.
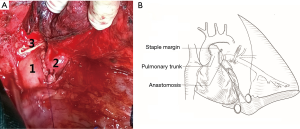
Airway conduit anastomosis between the main left and lower segment of the bronchi was performed with end-to-end 3-0 interrupted non-absorbable suture in a circle. A hole of a one-centimeter diameter was cut in the PA trunk after clamping with lateral forceps. The PA of the inferior lobe was translocated to the sidewall of the PA trunk, and end-to-side anastomosis was performed with a 5-0 running polypropylene suture (Figure 2A,B). Local administration of sodium heparin (5,000 IU/500 mL saline) was used for flushing and expulsion the air before final closure of the vessel anastomosis. Radical mediastinal lymph nodal dissection was performed after the reconstructions, and lymph node stations 4, 5, 6, 7, 8, 9, and 10 were removed.
Results
Tumor response of neoadjuvant chemotherapy
Two patients were re-evaluated with the CT scan-based clinical response assessment after two cycles of induction chemotherapy, indicating that they had achieved partial response to induction chemotherapy.
Surgical procedure and postoperative outcomes
Translocation of the PA of the left inferior lobe to the PA trunk with end-to-side anastomosis was performed, and additionally, sleeve bronchial resection with end-to-end anastomosis was accomplished. The median operative bleeding was 250 mL (range, 200–1,200 mL), and median operative time was 140 min (range, 110–180 min). Leakage of vascular anastomose with 1,200 mL operative bleeding occurred in the first case. Protamine was not administered in all patients at the end of the surgery.
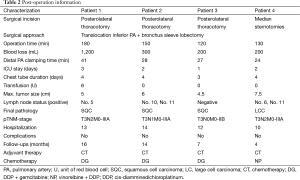
We observed no postoperative complication or mortality. Average length of hospital stay was 12.25 days (range, 10–14 days). Final pathology confirmed squamous carcinoma in three patients and adenocarcinoma in one patient, specifically, pathological N2 status was confirmed in 2 patients and N1 status in 1 patient. Post-operative pathological stage was confirmed as T3N2M0-IIIA in two cases, T3N1M0-IIIA in one case, and T3N0M0-IIB in one case (post-operation demographics are shown in Table 2).
Full table
Adjuvant therapy and follow-up information
Subcutaneous low-molecular-weight heparin (4,100 IU/d) was administered to all patients until 7 days post-operation. Chemotherapy was administered to all patients because of advanced pathologic status. Chemotherapy regimens consisted of six cycles of cisplatin 80 mg/m2 D1 and gemcitabine 1,200 mg/m2 on D1 and D8 (repeated every 21 days) in three patients. Four cycles of navelbine 25 mg/m2 on days 1 and 8 combined with Cisplatin 80 mg/m2 D1 was recommended for patient No. 4. Three patients had finished 6 cycles of chemotherapy, one patient had finished 4 cycles. Complete follow-up information was obtained for four cases, and the follow-ups ended on Oct. 21, 2015. All patients kept vascular patency by one month postoperative CT scans (Figure 3A-D).

Discussion
Translocation of the left inferior lobar PA to the PA trunk associated with a bronchial sleeve resection of the left superior lobe was performed in very unusual cases. The techniques described can be used when a left upper lobe tumor extensively infiltrates the left main PA beyond the arterial ligament, but with uninvolved bifurcation and distal of the PA. Bronchial sleeve resections were performed by removing the left upper lobes along with PA resection; The unusual situation (PA sleeve without bronchial sleeve) may produce a long bronchial segment separating the two widely spaced PA stumps, a prosthetic conduit should be used for the resected vascular segment (4,5). The origin of the left main PA was dissected in the pericardium and closed by auto-suture. End-to-end anastomosis between the orifice of the bifurcation of the PA and stump distal to the left PA was not feasible because the distance was too long, and vascular diameters did not match. The left inferior lobar PA can be translocated to the root of the PA trunk, and end-to-side anastomosis can be performed to avoid a pneumonectomy or prosthetic conduit replacement. For an advanced hilar tumor in the right superior lobe, a graft may be used as an acceptable lung-sparing technique because of the anatomical character of the PA (6-8).
Translocation of the left inferior lobar PA to the PA trunk is indicated when a tumor infiltrates the origin of left upper lobar bronchus and the initial segment of the left PA. The advantages of this technique are as follows: (I) avoiding tension on the anastomosis of reconstruction of left PA; (II) the diameter of the hole in PA trunk matches well with the diameter of distal to the left PA; and (III) the bronchial and vascular anastomoses are separated widely, minimizing the risk of PA erosion and consequent fatal bleeding in the case of bronchial anastomosis dehiscence or fistula (9). Posterolateral thoracotomy is preferable for performing complex bronchovascular reconstructions, median sternotomies may be performed in case of extensive invasion of mediastinum. The key aim of the procedure is to expose enough space in the PA bifurcation to close the orifice of the left main PA. The pericardium typically needs to be opened to access the pulmonary trunk and PA bifurcation. Cardiopulmonary bypass should be considered if there is no space to utilize auto-suture in the PA bifurcation (10). Sleeve resections of bronchi can be performed in a routine manner with end-to-end anastomosis. All of these procedures should include examination of all margins by frozen section to ensure R0 resection (11). In our series, median sternotomy was used in one patient due to mediastinal structure extensively invaded by large tumor. Four patients had operation without cardiopulmonary bypass and all reconstructed vessels retained patency with one month regular post-operative CT-scan.
Locally advanced bronchogenic carcinoma on the left beyond the arterial ligament is usually regarded as an unresectable disease, or it is often addressed by pneumonectomy or reconstruction of the left PA via cardiopulmonary bypass when a radical resection is undertaken (10). Pneumonectomy for these specially localized lung cancers, although technically simpler, may result in impaired lung function and even poorer survival. According to Meacci (12), patients with stage IIIa-N2 who underwent pneumonectomy or bilobectomy had worse overall survival and disease-free survival than did lobectomies (5-year survival: 28.3% for lobectomies vs. 0% of pneumonectomies). Broncho-angioplasty for lung cancer may offer favorable outcomes for strictly indicated cases. Several studies have suggested that complex lung-sparing operations can be pursued if complete anatomical resection is achieved. Chunwei et al. (13) documented 5- and 10-year survival rates with bronchoplasty, including PA reconstruction, in 21 patients at 33.3% and 16.7%, respectively. Icar et al. (14) demonstrated that 5-year actuarial survival of bronchial sleeve lobectomy associated with arterial resection was 9%, and for PN1 cases, 1-, 2-, and 3-year survival rates were 81%, 68%, and 31%, respectively. Although the 5-year survival of bronchial sleeve lobectomy associated with an arterial resection was significantly lower compared to that of single bronchoplastic, it is most applicable for indicated cases, given that the median survival time (MST) is only 12 months for inoperable locally advanced NSCLC patients (15).
However, various types of operations for locally advanced lung cancers are still being debated (16). A retrospective analysis of N2 patients with SVC infiltrate indicated that these patients experienced survival rate comparable to the survival rate of individuals with N1–0 disease after induction therapy (17). Icar et al. reported that two of three patients with pN2 who underwent sleeve lobectomy with associated arterial resection died within seven years of the operation (14). Five-year survival was 30.5% for N2 NSCLC patients with surgical intervention and only 22.2% for patients undergoing chemotherapy, and 27% for patients undergoing radiotherapy alone (18). As an essential component of multimodality therapeutic approaches, surgery may provide favorable survival for resectable N2 patients.
Our study has several limitations. It was a retrospective study, which included only four cases. Additionally, the longest postoperative observed duration was only 16 months; therefore, a larger number of cases and longtime follow-up are required to evaluate the safety of this operation process and long-term oncologic results. However, translocation of left inferior lobar PA to PA trunk is a feasible technique to avoid a pneumonectomy in patients with left locally advanced lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Suen HC, Meyers BF, Guthrie T, et al. Favorable results after sleeve lobectomy or bronchoplasty for bronchial malignancies. Ann Thorac Surg 1999;67:1557-62. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 1976-7.
- Read RC, Ziomek S, Ranval TJ, et al. Pulmonary artery sleeve resection for abutting left upper lobe lesions. Ann Thorac Surg 1993;55:850-4; discussion 853-4. [Crossref] [PubMed]
- Ibrahim M, Maurizi G, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. Thorac Surg Clin 2013;23:337-47. [Crossref] [PubMed]
- Solli P, Spaggiari L, Grasso F, et al. Double prosthetic replacement of pulmonary artery and superior vena cava and sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg 2001;20:1045-8. [Crossref] [PubMed]
- Pochesci I, Ibrahim M, Vismara LG, et al. Superior vena cava replacement by the stapled pericardial conduit associated with double sleeve resection of the bronchus and pulmonary artery. Eur J Cardiothorac Surg 2008;34:673. [Crossref] [PubMed]
- Sekine Y, Yasufuku K, Motohashi S, et al. Triple reconstruction of pulmonary artery, superior vena cava and bronchus for lung cancer. Interact Cardiovasc Thorac Surg 2006;5:509-10. [Crossref] [PubMed]
- D'Andrilli A, Venuta F, Maurizi G, et al. Bronchial and arterial sleeve resection after induction therapy for lung cancer. Thorac Surg Clin 2014;24:411-21. [Crossref] [PubMed]
- Pope NH, Ailawadi G. Transcatheter Mitral Valve Repair. Oper Tech Thorac Cardiovasc Surg 2014;19:219-37. [Crossref] [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Lung conservation techniques: bronchial sleeve resection and reconstruction of the pulmonary artery. Semin Surg Oncol 2000;18:165-72. [Crossref] [PubMed]
- Meacci E, Cesario A, Cusumano G, et al. Surgery for patients with persistent pathological N2 IIIA stage in non-small-cell lung cancer after induction radio-chemotherapy: the microscopic seed of doubt. Eur J Cardiothorac Surg 2011;40:656-63. [PubMed]
- Chunwei F, Weiji W, Xinguan Z, et al. Evaluations of bronchoplasty and pulmonary artery reconstruction for bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:209-13. [Crossref] [PubMed]
- Icard P, Regnard JF, Guibert L, et al. Survival and prognostic factors in patients undergoing parenchymal saving bronchoplastic operation for primary lung cancer: a series of 110 consecutive cases. Eur J Cardiothorac Surg 1999;15:426-32. [Crossref] [PubMed]
- Hotta K, Kiura K, Fujiwara Y, et al. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PLoS One 2011;6:e26646. [Crossref] [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
- Shargall Y, de Perrot M, Keshavjee S, et al. 15 years single center experience with surgical resection of the superior vena cava for non-small cell lung cancer. Lung Cancer 2004;45:357-63. [Crossref] [PubMed]
- Bakir M, Fraser S, Routledge T, et al. Is surgery indicated in patients with stage IIIa lung cancer and mediastinal nodal involvement? Interact Cardiovasc Thorac Surg 2011;13:303-10. [Crossref] [PubMed]