Will testosterone replacement therapy become a new treatment of chronic heart failure? A review based on 8 clinical trials
Introduction
Chronic heart failure (CHF) is a growing health problem throughout the world, especially on ageing Western population. The prevalence of CHF in the UK was 1% and in Europe alone around 10 million people were affected by CHF. Despite modern medicine advanced in the respect of detection, diagnosis and treatment of CHF, the prognosis of this condition was still poor and worse than the prognosis of most malignancies. CHF is a syndrome characterized by an anabolic-catabolic imbalance of both peripheral skeletal muscles and heart which might involve neurohumoral, endocrine and metabolic systems. Impairment of major anabolic systems may be thought involved widely in the CHF pathophysiology. Especially low serum testosterone levels have been correlated to the symptoms severity and adverse outcomes in the CHF. Therefore, it is necessary to understand whether it is effective for testosterone replacement therapy (TRT) in patients with CHF. The existing researches have not yet demonstrated the physiopathologic mechanism and effectiveness of TRT clear. But some recent evidence has emerged that TRT could improve muscle strength, exercise tolerance, functional pulmonary capacity, insulin sensitivity, Beck depression inventory, and adjust the neuroendocrine factors in patients with CHF. But in respect of echocardiographic examination and inflammatory markers, the results from different researches were controversial. The purpose of this review was to sum up the available evidence that testosterone was deficient in patients with CHF as well as TRT was good to patients with CHF, and there were not the potential side effects of TRT (1,2).
Materials and methods
Identification of eligible studies
One search strategy was searched using the search terms “testosterone” and “heart failure” with no limitations. In addition, another search strategy was also conducted by using the terms “testosterone” and “heart failure” limited to “humans”, “clinical trial”. A broad search of the English-language literatures for randomized controlled trials (RCTs) in patients with CHF was performed by using PubMed, Medline, Cochrane Central Register of Controlled Trials, Web of Science, and trial registry (e.g., ClinicalTrials.gov website) databases. All the relevant publications were reviewed, and duplications of articles from the two search strategies were eliminated. The articles in reference lists were also hand-searched for potentially relevant publication. The search was conducted by two investigators. Any disagreements were resolved by consensus with involvement of the third author (3).
Inclusion and exclusion criteria
All human-associated studies, regardless of the year of publication, would be included if they met the following criteria: RCT, the age of participators was over 18 years old, clinically stable CHF without hospital admission for heart failure in previous 3 months before recruitment, evidence of impairment of left ventricular systolic function [ejection fraction (EF) ≤40%], reduced exercise tolerance associated with breathlessness of cardiac origin, symptomatic heart failure with New York Hear Association (NYHA) functional class II/III/IV, and sufficient data of clinical outcomes. All the studies would exclude if they met the following criteria: animal experiment, review, mechanism research, case report, collection of papers, literatures of the incomplete data and duplicate, and not obtain full manuscripts. Participants would be excluded if they had unstable angina, recent acute myocardial infarction (AMI), decompensated heart failure, hemodynamically significant valvular heart disease, uncontrolled hypertension, renal insufficiency (serum creatinine ≥200 μmol/L), orthopedic or neurologic illness which limited the ability to exercise, prostate cancer, prostate-specific antigen level above the age-adjusted reference range, already have received sex hormone therapy, and allergy to peanut or soya (4).
Data extraction
Two investigators extracted data independently and reached a consensus on all the items. For each study, the following information was collected: first author, the year of publication, sample size, mean age, gender, heart failure status, NYHA class, left ventricular EF (LVEF), testosterone formulation used, trial duration and clinical outcomes. The clinical outcomes included exercise capacity which was measured by using shuttle walk distance (SWD) and 6-min walk distance (6MWD), hemodynamic parameters described by SBP and DBP, electrocardiogram indicators including heart rate (HR), corrected Q-T intervals (Q-Tc interval) and Q-T interval dispersion (Q-Td interval), muscle strength measured by handgrip strength, isokinetic power torque (PTmax) and maximal voluntary contraction (MVC), echocardiography guidelines described by ejection faction (EF), and laboratory indexes measured by NT-proBNP, TNF-α, hs-CRP and IL-6. Data could be extracted separately as long as there was enough information in the trials.
Results
Literature search
A total of 229 articles (all published) were retrieved from the databases. A total of 50 articles from animal experiment, 63 articles from review, 7 articles from letter, 8 articles from case report, 21 articles from non-RCTs, 29 articles without the full text, 40 unrelated articles, and 3 articles with incomplete data were excluded. Thus, a total of remaining 8 publications met criteria for inclusion and exclusion, and details from the trials were extracted separately. Figure 1 showed a flowchart of article selection and inclusion. Due to the heterogeneity of patients, administration methods, a large variety of outcome measurement used in these trials, pooling of data for meta-analysis was inappropriate. Results were, therefore, summarized qualitatively.
Study characteristics
Details from 8 eligible trials published are analyzed in Table 1. Table 1 summarized the characteristics of the 8 trials. The number of participators in these trials ranged from 20 to 84, with the median age from 60 to 70.35 years, and all the participants’ gender was male except one study. Trial duration ranged from 3 to 6 months, and testosterone formulation used including intramuscular injection (IM), transdermal drug delivery, and androderm. The EF of all patients with stable CHF in our study was less than 40%.

Full table
Clinical outcomes
Effect of TRT on exercise capacity
Table 2 showed that TRT could improve significantly the exercise capacity of patients, compared with placebo. A total of 6 trials in Table 2 demonstrated that TRT group had shown significant improvement on 6MWD or SWD from baseline in CHF patients, compared with placebo. According to Mirdamadi et al. (4), those who received testosterone had a significant increasing trend in 6MWD parameter within the study period (6MWD at baseline was 407.44±100.23 m and after 12 weeks of follow-up reached 491.65±112.88 m following testosterone therapy, P=0.019). In the study of Malkin et al. (9), the mean change in SWDs at 12 months was 25±15 meters improvement from baseline. As well, in the researches of Iellamo et al. (7), Caminiti et al. (8) and Pugh et al. (10), distance walked at the 6MWD or SWD improved in both groups, but the increase was significant only in patients under testosterone supplementation. Stout et al. (5) found out that both the placebo group and TRT group revealed significant improvement on maximum walking distance in men with CHF.
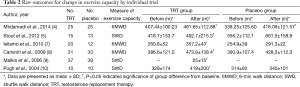
Full table
Effect of TRT on hemodynamic parameters
Total of 4 trials have involved hemodynamic parameters measured by SBP and DBP in Table 3. In the study of Mirdamadi et al. (4), no significant differences were revealed in the trend of the changes in hemodynamic parameters including systolic and diastolic blood pressures (DBPs) as well as HR between the two groups during the 12-week study period. Iellamo et al. (7) found that no significant changes in HR, or systolic and DBP were detected in either group. But Caminiti et al. (8) reported that both groups showed a tendency toward BP decrease, with significant results only for DBP in the TRT group. However, in the trial of Malkin et al. (9), SBP remained stable over the follow-up period in those on testosterone but fell in those on placebo (difference P=0.013). Thus it could be seen that the effect of TRT on SBP and DBP was controversial. So there was a need for high-quality studies to make us better understand the clinical effects of testosterone.
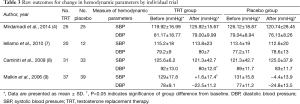
Full table
Effect of TRT on electrocardiogram indicators
According to Schwartz et al. (6), raw Q-T intervals were longer in women compared with men at baseline (P<0.03), whereas HRs did not differ, resulting in a trend towards longer Q-Tc intervals in women compared with men. Testosterone decreased Q-T intervals compared with placebo in both men and women (see Table 4), but did not significantly affect HR; thus, Q-Tc interval trends were similar to raw Q-T changes. The magnitude or absolute Q-Tc changes appeared similar in both men and women. Malkin et al. (11) found that in men with congestive heart failure, testosterone reduced the Q-Td, whereas placebo had no effects. But the 5 trials demonstrated TRT had no impact on HR in Table 4.
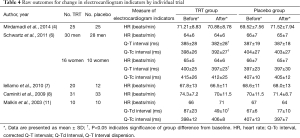
Full table
Effect of TRT on muscle strength
The MVC and PTmax were significantly improved in TRT patients but remained unchanged in the placebo group, according to Iellamo et al. (7) and Caminiti et al. (8) (Table 5). In the study of Malkin et al. (9), handgrip strength improved significantly with testosterone treatment. Between the remaining trials of Mirdamadi et al. (4) and Stout et al. (5), the muscle strength was gradually increased in either group; however, this trend was not different across the two groups.

Full table
Effect of TRT on echocardiography guidelines
According to EF assessed by echocardiography, no differences were observed between the patients who were prescribed testosterone and those who received placebo from baseline to end of the study time among the 4 trials of Mirdamadi et al. (4), Iellamo et al. (7), Caminiti et al. (8) and Malkin et al. (9) (Table 6).

Full table
Effect of TRT on laboratory indexes
A total of 2 trials related to the effect of TRT on laboratory indexes in Table 7 [Stout et al. (5) and Malkin et al. (9)]. There were no differences between the groups in circulating levels of NT pro-BNP, and inflammatory markers were unchanged, except for a decrease in TNF-α from baseline in the placebo group from the study of Stout et al. (5). Malkin et al. (9) demonstrated that there were no significant changes in serum level of BNP or TNF-α (Table 7).
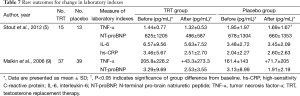
Full table
Side effect
Testosterone supplementation therapy appeared to be safe, and the subjects who accepted testosterone treatment did not appear any obvious adverse reactions. Long-term concerns that testosterone treatment might improve functional capacity and quality of life which were not apparent within the boundaries of physiological replacement, and in fact there was substantial evidence that this might be beneficial. Fluid retention, listed as an acknowledged side effect of testosterone treatment, was very rarely seen. There were realistic concerns over the risk of prostate malignancy. Prostatic malignancy should be excluded prior to commencing testosterone therapy by measurement of prostate specific antigen and digital examination (12-14).
Discussion
The fact that CHF was characterized by disordered metabolism, reduced anabolic function. Most patients suffered a gradual decline in muscle mass, strength and endurance that reflected from the maladaptive imbalance and a relative deficiency of anabolic hormones (1). It has been found that relative testosterone deficit reflected aspect of anabolic in sufficiency that led to a metabolic shift favoring catabolism, a major underlying mechanism for tissue wasting seen in CHF (1,2). That testosterone deficiency was a precursor to the development of CHF or a consequence of the condition or a combination of both, which were not clear at present (15-17).
This article revealed that testosterone supplementation in patients with CHF was associated with an improvement in exercise capacity, muscle strength and electrocardiogram indicators, but no significant changes in EF, SBP, DBP, NT-proBNP, TNF-α and inflammatory markers. Some of them were controversial, such as SBP, DBP, Q-Tc interval and TNF-α. Reasons for dispute were related to the difference of trial duration, testosterone formulation used, methods and time frames of measurement, testosterone existence forms, heterogeneity of patients and so on. So there was a need for high-quality studies to make us better understand the clinical effects of testosterone. Total testosterone was composed of about 2% free testosterone, 50–60% testosterone combines with sex hormone binding globulin and 30–40% testosterone combines with albumin. DHEA was needed to serve as a precursor of testosterone synthesis. So the different testosterone existence forms could result in different results and errors (18). But beyond that, methods and time frames of measurement could also influence the results of trials.
Stout et al. (5) found out that both the placebo group and TRT group revealed significant improvement on maximum walking distance in men with CHF. The reason why both the placebo group and TRT group showed significant effects on maximum walking distance were whether placebo group or TRT group was doing exercise rehabilitation in the trial that Stout et al. (5) showed. Exercise rehabilitation has been proved to improve maximum walking distance in men with CHF (19). Testosterone supplementation group of men patients with CHF rather than women with CHF or placebo group, shortened Q-Tc interval without HR changes according to Schwartz et al. (6) (see Table 4). However, Malkin et al. (11) certified no significant changes in testosterone supplementation group or placebo group in the aspect of Q-Tc interval in men patients with CHF. Furthermore, TRT group reported significant shortening in terms of Q-Td Interval in men with CHF, but that was not apparent in the placebo group (11). Causes for the different results between the two trials (6,11) included in homogeneity of considered trial duration, methods of Q-Tc interval measurement and so on.
There were only 2 trials of Schwartz et al. (6) and Iellamo et al. (7) described women patients with CHF from 8 eligible trials. It addressed the potential benefits of testosterone administration in women with CHF for the first time. Finally, we obtained the results that testosterone supplementation treatment improved maximum walking distance and muscle strength in women patients with CHF. The benefits of testosterone administration in women with CHF needed a large of high-quality studies to better verify clinical effects of testosterone in women with CHF.
The use of testosterone in heart failure has not filtered into widespread clinical practice, because the limited evidence of evidence-based medicine and there was also considerable anxiety and suspicion of cardiac specialists, in particular to prescribe a male sex hormone to treat a cardiac condition. In this area, endocrinologists may need to take a lead (20). Toma et al. (15) put forward in their study that testosterone appeared to be a promising therapy to improve functional capacity in patients with HF. Furthermore, in their study, adequately powered RCTs were required to assess the benefits of testosterone in high-risk population with regard to quality of life, clinical events, and safety. It provided us with strong evidence for a follow-up study. This article carried out a review based on 8 clinical trials to find out that TRT has emerged as a possible therapeutic option and small prospective clinical trials have shown promising results in improving functional capacity and quality of life. All the trials demonstrated that TRT appeared to be safe, and the subjects who accepted testosterone treatment did not appear any serious adverse reactions. Although there were controversial results in terms of clinical outcomes, most of the study evidence indicated that TRT was effective in CHF. There appeared to be no reason to restrict testosterone replacement to men with CHF since the limited evidence showed that benefit was accrued.
Conclusions
All in all, TRT has emerged as a possible therapeutic option and prospective clinical trials have shown promising results in improving functional capacity and quality of life. But it is necessary to exclude prostate cancer before treatment with testosterone (21). There was also a concern regarding the long-term risk of prostate cancer with testosterone treatment. This issue remained unanswered at present, primarily because large, long-term prospective trials of testosterone therapy were absent. However, several problems, such as the risks of long-term high-dose TRT, remained to be clarified.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81372035, 81571873), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, Jx10231081), the Foundation of the Health Department of Jiangsu Province (H201301), the Six Talents Peak Project of Jiangsu Province (2013WSN035), the Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMKF201311, 201408), Lijieshou intestinal barrier research fund (LJS-201306), the general financial from the China Postdoctoral Science Foundation (2014M561735), Key Project supported by Medical Science and technology development Foundation, Jiangsu (BL2014082).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Malkin CJ, Channer KS, Jones TH. Testosterone and heart failure. Curr Opin Endocrinol Diabetes Obes 2010;17:262-8. [Crossref] [PubMed]
- Malkin CJ, Jones TH, Channer KS. Testosterone in chronic heart failure. Front Horm Res 2009;37:183-96. [Crossref] [PubMed]
- Qiu T, Zhou L, Zhu W, et al. Effects of treatment with histone deacetylase inhibitors in solid tumors: a review based on 30 clinical trials. Future Oncol 2013;9:255-69. [Crossref] [PubMed]
- Mirdamadi A, Garakyaraghi M, Pourmoghaddas A, et al. Beneficial effects of testosterone therapy on functional capacity, cardiovascular parameters, and quality of life in patients with congestive heart failure. Biomed Res Int 2014;2014:392432.
- Stout M, Tew GA, Doll H, et al. Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J 2012;164:893-901. [Crossref] [PubMed]
- Schwartz JB, Volterrani M, Caminiti G, et al. Effects of testosterone on the Q-T interval in older men and older women with chronic heart failure. Int J Androl 2011;34:e415-21. [Crossref] [PubMed]
- Iellamo F, Volterrani M, Caminiti G, et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol 2010;56:1310-6. [Crossref] [PubMed]
- Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol 2009;54:919-27. [Crossref] [PubMed]
- Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J 2006;27:57-64. [Crossref] [PubMed]
- Pugh PJ, Jones RD, West JN, et al. Testosterone treatment for men with chronic heart failure. Heart 2004;90:446-7. [Crossref] [PubMed]
- Malkin CJ, Morris PD, Pugh PJ, et al. Effect of testosterone therapy on QT dispersion in men with heart failure. Am J Cardiol 2003;92:1241-3. [Crossref] [PubMed]
- Morgentaler A, Miner MM, Caliber M, et al. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 2015;90:224-51. [Crossref] [PubMed]
- Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res 2014;43:1-20. [PubMed]
- Tirabassi G, Gioia A, Giovannini L, et al. Testosterone and cardiovascular risk. Intern Emerg Med 2013;8 Suppl 1:S65-9. [Crossref] [PubMed]
- Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail 2012;5:315-21. [Crossref] [PubMed]
- Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail 2007;9:44-50. [Crossref] [PubMed]
- Saxton JM, Stout M. Exercise and testosterone supplementation in male chronic heart failure patients with low testosterone status. Am Heart J 2013;166:e23. [Crossref] [PubMed]
- Pugh PJ, Jones RD, Malkin CJ, et al. Physiologic testosterone therapy has no effect on serum levels of tumour necrosis factor-alpha in men with chronic heart failure. Endocr Res 2005;31:271-83. [Crossref] [PubMed]
- Saxton JM, Zwierska I, Mathur A, et al. Study protocol to investigate the effects of testosterone therapy as an adjunct to exercise rehabilitation in hypogonadal males with chronic heart failure. BMC Cardiovasc Disord 2006;6:46. [Crossref] [PubMed]
- Bušić Z, Culić V. Testosterone treatment and exercise capacity. Am Heart J 2013;166:e21. [Crossref] [PubMed]
- Edelman S, Butler J, Hershatter BW, et al. The effects of androgen deprivation therapy on cardiac function and heart failure: implications for management of prostate cancer. Clin Genitourin Cancer 2014;12:399-407. [Crossref] [PubMed]



