
Epidemiology of respiratory syncytial virus in hospitalized children with community-acquired pneumonia in Guangzhou: a 10-year study
Highlight box
Key findings
• The detection rate of RSV in CAP hospitalized children changed by years, months, ages, and sexes. CAP hospitalized children with RSV are more likely to develop severe pneumonia than those without RSV.
What is known and what is new?
• The epidemiology characteristics of RSV in hospitalized children has been reported in western countries.
• We report the epidemiology of RSV in hospitalized children with CAP in China, and investigated the relationship between RSV and severe pneumonia.
What is the implication, and what should change now?
• Policy makers and doctors should make timely adjustments to prevention measures, medical resources and treatment options based on these epidemiological characteristics. Further prospective randomized studies are needed.
Introduction
Community acquired pneumonia (CAP) is one of the most common causes of hospitalization and death in children younger than 5 years (1). According to a previous study, pneumonia kills over 2 million children worldwide every year, which accounts for more deaths of young children than any other single infectious disease, such as malaria, diarrhea and dengue (2).
Respiratory syncytial virus (RSV) is the most infectious viral pathogen that causes CAP in children under 5 years of age (3). Moreover, RSV infection is the primary cause of hospitalization for viral respiratory infection in infants and young children, which seriously endangers children’s health (4). However, there are few reports on the epidemiology of RSV infection in large samples of hospitalized children with CAP.
To understand the epidemiological characteristics of RSV infection in hospitalized children with CAP in Guangzhou and guide the prevention and treatment of RSV infection, we analyzed the epidemiological characteristics of RSV infection in 9,837 hospitalized children with CAP. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-331/rc).
Methods
Study population
A total of 10,216 hospitalized children (≤14 years old) who were diagnosed with CAP according to the criteria of the Child Community-Acquired Pneumonia Guidelines (I, II) (5,6) in our hospital from January 2010 to December 2019 were included in our study. The exclusion criteria were as follows: (I) children with missing demographic data and (II) children without RSV test results. Severe CAP was defined as follows: (I) fever ≥38.5 ℃; (II) respiratory rate greater than 70 breaths per minute in infants and 50 breaths per minute in children (except while crying); (III) cyanosis or intermittent apnea; and (IV) dehydration or confusion (5,6).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First Affiliated Hospital of Guangzhou Medical University Ethics Committee (No. 20170315), and individual consent for this retrospective analysis was waived.
Data and specimen collection
Oropharyngeal swab samples were collected on the day of admission or in the early morning of the second day. At the same time, basic information such as sex and age were collected. The oropharyngeal swab samples were put into a 15-mL tube containing 2.5 mL of virus delivery solution and transported to the State Key Laboratory of Respiratory Diseases at a temperature of 2–8 ℃. The samples were analyzed immediately, and the remaining samples were stored in another cryotube at −80 ℃.
Laboratory testing
In brief, DNA or RNA from oropharyngeal swab samples was extracted using QIA amp DNA Mini Kit or QIA amp Viral RNA Mini Kit (Qiagen Co. Ltd., Shanghai, China) in accordance with the manufacturer’s protocols. For RSV detection, the primers and TaqMan probes were designed to detect RSV subgroups A (RSVA) and B (RSVB) by amplifying the RSV G gene. Multiplex real-time reverse transcriptase (RT)-PCR was conducted using our optimized reaction buffer and cycling conditions. The cycling conditions were 48 ℃ for 10 min, 94 ℃ for 2 min, and then 40 cycles of 94 ℃ for 10 s and 55 ℃ for 35 s. The amplified nucleic acids were detected with the Applied Biosystems 7500 Real-Time PCR System (Life Technologies, Singapore). RSV-positive samples were tested simultaneously for 9 respiratory tract pathogens, influenza virus A (INFA), influenza virus B (INFB), parainfluenza virus (PIV), enterovirus (EV), coronavirus (CoV), human metapneumovirus (HMPV), human bocavirus (HBoV), human rhinovirus (HRV), and adenovirus (ADV) using kits from Guangzhou HuYanSuo Medical Technology Co., Ltd. To ensure sequence accuracy, PCR amplification and sequencing were conducted at least twice. The detailed testing procedure has been provided in previous reports (7,8).
Statistical analysis
SPSS 25.0 statistical software (IBM Corp. Released in 2017. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY) was used for the analysis. The detection rate of viruses was calculated by dividing the number of positive cases by the total case number, which was expressed as a percentage. Line charts were drawn to describe changes in the epidemiological characteristics of viruses. The chi-square test or Fisher’s exact test was used for the comparison of categorical data. A multivariable logistic regression model which adjusted for sex, age, month and coinfection was used to assess the association of severe pneumonia and death with RSV infection with the calculation of odds ratio (OR) and 95% confidence interval (CI). A multivariable logistic regression model which adjusted for sex, age and month was used to assess the association of severe pneumonia and coinfection. In addition, the association between severe pneumonia and cycle threshold (CT) values of RSV were examine using Pearson correlation analysis in RSV-positive children, with the calculation of Pearson correlation coefficient (r).
Results
Patient characteristics and detection rate of RSV
In this study, we excluded 379 patients without complete demographic data or RSV test results, and a total of 9,837 oropharyngeal swabs from hospitalized children with CAP were included (Figure 1). There were 6,226 males and 3,611 females. The total RSV detection rate was 15.3% (1,507/9,837). RSV-positive children were significantly younger than RSV-negative children (1.49±1.78 vs. 2.86±2.83, P<0.001) (Table 1).
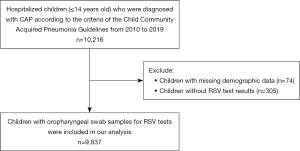
Table 1
| Variables | RSV-positive | RSV-negative | P value |
|---|---|---|---|
| N | 1,507 (15.3) | 8,330 (84.7) | <0.001 |
| Age, mean ± SD, years | 1.49±1.78 | 2.86±2.83 | |
| Age range, years | |||
| ≤0.5 | 410 (24.5) | 1,261 (75.5) | <0.001 |
| (0.5–1] | 484 (19.8) | 1,966 (80.2) | <0.001 |
| (1–3] | 470 (16.3) | 2,410 (83.7) | <0.001 |
| (3–6] | 106 (5.8) | 1,711 (94.2) | <0.001 |
| >6 | 37 (3.6) | 982 (96.4) | <0.001 |
| Sex | |||
| Male | 1,024 (16.4) | 5,202 (83.6) | <0.001 |
| Female | 483 (13.4) | 3,128 (86.6) | <0.001 |
| Year | <0.001 (P for trend) | ||
| 2010 | 81 (11.5) | 623 (88.5) | |
| 2011 | 158 (24.8) | 478 (75.2) | |
| 2012 | 113 (12.3) | 803 (87.7) | |
| 2013 | 131 (10.0) | 1,181 (90.0) | |
| 2014 | 203 (16.5) | 1,026 (83.5) | |
| 2015 | 171 (20.7) | 657 (79.3) | |
| 2016 | 131 (16.5) | 665 (83.5) | |
| 2017 | 145 (20.3) | 571 (79.7) | |
| 2018 | 223 (18.9) | 956 (81.1) | |
| 2019 | 151 (9.9) | 1,370 (90.1) | |
| Month | <0.001 (P for trend) | ||
| January | 170 (19.4) | 708 (80.6) | |
| February | 123 (25.5) | 359 (74.5) | |
| March | 203 (21.0) | 762 (79.0) | |
| April | 169 (18.4) | 751 (81.6) | |
| May | 136 (14.2) | 823 (85.8) | |
| June | 88 (10.9) | 719 (89.1) | |
| July | 130 (15.2) | 728 (84.8) | |
| August | 138 (15.8) | 738 (84.2) | |
| September | 141 (18.7) | 615 (81.3) | |
| October | 61 (8.8) | 629 (91.2) | |
| November | 49 (6.0) | 769 (94.0) | |
| December | 99 (12.0) | 729 (88.0) | |
| Severe pneumonia | 180 (11.9) | 757 (9.1) | 0.194 |
| Death | 3 (0.2) | 43 (0.5) | 0.100 |
Values are number and frequency (%) of total unless otherwise specified. RSV, respiratory syncytial virus; SD, standard deviation.
Correlation between the detection rate of RSV and patient demographics
The detection rate of RSV in male children (1,024/6,266, 16.4%) was significantly higher than that in female children (483/3,611, 13.4%) (P<0.001). The detection rate of RSV in hospitalized children with CAP was different in every age group (χ2=382.137, P<0.001). The detection rate of RSV was highest in children younger than 0.5 years old (410/1,671, 24.5%). The younger the patient was, the higher the detection rate of RSV (Figure 2).

Annual and monthly changes in the detection rate of RSV
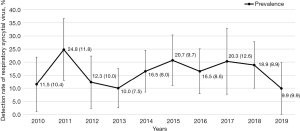
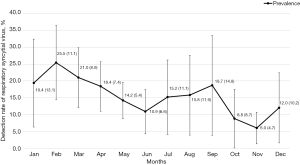
From 2010 to 2019, the detection rate of RSV fluctuated (χ2=166.982, P<0.001), with the highest detection rate in 2011 (158/636, 24.8%), followed by 2015 (171/828, 20.7%). The year with the lowest detection rate was 2019 (151/1,521, 9.9%) (Figure 3). The detection rate of RSV was different in different months (χ2=184.740, P<0.001). The highest detection rate was in February (123/482, 25.5%), followed by March (203/965, 21.0%) and January (170/878, 19.4%). The lowest detection rate was in November (49/818, 6.0%) (Figure 4).
Distribution of RSV in children with severe pneumonia
Children with severe pneumonia accounted for 9.5% (937/9,837). After adjusting for potential confounders, the RSV-positive children were associated with increased risk of severe pneumonia compared to RSV-negative children (multivariable-adjusted OR 1.26, 95% CI: 1.04 to 1.53, P=0.019). There were 3 (3/1,507, 0.2%) RSV-positive and 43 (43/8,330, 0.5%) RSV-negative hospitalized children with CAP who died during our study, but the difference was not statistically significant (multivariable-adjusted OR 0.27, 95% CI: 0.07 to 1.09, P=0.066) (Table 2). In addition, children with severe pneumonia had significantly lower CT values than those without severe pneumonia (28.88±3.89 vs. 30.42±3.33, P<0.01), suggesting higher concentrations of the virus in children with severe pneumonia. The Pearson correlation analysis indicated the significantly negative association between CT values and severe pneumonia (r=−0.146, P<0.01).
Table 2
| Complications | RSV positive | RSV negative | P value |
|---|---|---|---|
| Severe pneumonia | |||
| Crude OR (95% CI) | 1.36 (1.14, 1.61) | 1.00 (reference) | <0.001 |
| Multivariable-adjusted OR (95% CI)* | 1.26 (1.04, 1.53) | 1.00 (reference) | 0.019 |
| Death | |||
| Crude OR (95% CI) | 0.38 (0.12, 1.24) | 1.00 (reference) | 0.110 |
| Multivariable-adjusted OR (95% CI)* | 0.27 (0.07, 1.09) | 1.00 (reference) | 0.066 |
*, multivariable models were adjusted for age, sex, month and coinfection. RSV, respiratory syncytial virus; OR, odds ratio; CI, confidence interval.
The detection rate of coinfections
Among RSV-positive hospitalized children with CAP, 266 cases (266/1,507, 17.7%) exhibited coinfection with other viruses (Table 3). The most common coinfection was multiple coinfection (85/266, 32.0%), which was defined as coinfection with more than two viruses in addition to RSV. INFA (41/266, 15.4%) was the most common coinfection virus. Patients with coinfection (38/266, 14.3%) have a higher risk of severe pneumonia than those without coinfection (142/1,241, 11.4%), but the difference was not statistically significant (multivariable-adjusted OR 1.39, 95% CI: 0.94 to 2.05, P=0.101).
Table 3
| Type of virus | N (%) |
|---|---|
| INFA | 41 (15.4) |
| INFB | 10 (3.8) |
| PIV | 15 (5.6) |
| EV | 20 (7.5) |
| CoV | 28 (10.5) |
| HMPV | 6 (2.3) |
| HBoV | 14 (5.3) |
| HRV | 24 (9.0) |
| ADV | 23 (8.6) |
| Multiple infection* | 85 (32.0) |
*, multiple infection was defined as coinfection with more than two viruses in addition to RSV. INFA, influenza virus A; INFB, influenza virus B; PIV, parainfluenza virus; EV, enterovirus; CoV, coronavirus; HMPV, human metapneumovirus; HBoV, human bocavirus; HRV, human rhinovirus; ADV, adenovirus.
Discussion
Our data indicated that the detection rate of RSV was highest in hospitalized children with CAP ≤0.5 years old, and the detection rate of RSV gradually decreased with increasing age, which was consistent with a previous study on RSV epidemiology (9). The specific reasons why infants ≤0.5 years old were more likely to be infected with RSV may be ascribed to their immature lungs and immune system (10). The immune response at that stage usually has functional defects and quantitative defects of antigen-presenting cells and effector cells (11), which is different from the adult immune response. Thus, infants are susceptible to a variety of respiratory viruses, such as RSV (10), influenza virus (12) and measles (13). In addition, we found that the detection rate of RSV in male children was significantly higher than that in female children (P<0.001), suggesting that male children are relatively more likely to be infected with RSV. Similar to the results in our study, Radhakrishnan et al. found that there were more males than females (63.8% vs. 44.2%) children who were hospitalized for RSV infection in a population-based study of children in Ontario, Canada (14). Uekert et al. also found that sex differences were correlated with either the frequency or severity of viral respiratory tract infections during the first few years of life (15). Sex differences in corticosteroid secretion and activity could theoretically explain variations in susceptibility to RSV infection, and there is conclusive evidence that sex hormones can influence the development of specific lymphocyte populations and cytokine production (16,17).
The detection rate of RSV in hospitalized children with CAP was 15.3% in our study, which was lower than that in northern China (33.3%) (18) and higher than that in African countries, such as Kenya (8.1%) (19). In addition, the detection rates of RSV peaked in February, January and March in our study, which was consistent with the results of a previous study (20). Cui et al. (21) found that the RSV infection rate in Beijing was highest from November to February. Guangzhou is subtropical and has a lower latitude than Beijing, so winter comes later in Guangzhou than in Beijing, which may explain why the month with the highest RSV detection rate in our study was later than that in the study by Cui et al. (21). In cold months, the virus has increased vitality because of less light and children’s weakened immunity, which increase the risk of infection (22). In addition, increased gathering in the house during winter may also increase the risk of virus spreading.
We also found that the detection rate of RSV in hospitalized children with CAP from 2010 to 2019 exhibited a fluctuation every three or four years. A possible reason is the increased immunity for the prevalent RSV subgroup in children after a period of time, which may lead to an alternating prevalence between RSVA and RSVB (18,23). Song et al. (23) found that RSVB predominated between 2008 and 2010 in China, whereas RSVA predominated between 2010 and 2012. Previous studies also suggest that new mutant genotypes have been produced continuously since the BA genotype strain of RSVB was discovered in 2003 (24,25). By 2018, 15 genotypes derived from BA had been identified that could break through the previously established immunity for the BA genotype (24,25). However, more studies are needed to confirm the correlation between the infection rate of RSV in different years and various circulating genotypes.
The RSV-positive children were associated with increased risk of severe pneumonia compared to RSV-negative children (multivariable-adjusted OR 1.26, 95% CI: 1.04 to 1.53, P=0.019), which was consistent with other studies (26,27). These studies suggested that RSV infection is more likely to develop into severe pneumonia. The pathogenic mechanism of RSV infection is more complicated and involves the combined effects of causative factors, airway epithelial cell-related factors, immune system responses, nervous system responses, host factors, and environmental factors, which may worsen the child’s illness (28). We also found the significantly negative association between CT values and severe pneumonia, suggesting that children with higher concentrations of RSV may be prone to have severe pneumonia. This finding would help clinicians identify children at high risk of pneumonia early and make proper management strategies.
In this study, 17.7% (266/1,507) of hospitalized children with CAP who were RSV positive were also positive for other viruses. Moreover, patients with coinfection (38/266, 14.3%) tended to have a higher incidence of severe pneumonia than those without coinfection (142/1,241, 11.4%), but the difference was not statistically significant (P=0.101). Some scholars have reported that compared with single RSV infection, RSV coinfection with other viruses had higher rates of pneumonia, hospitalization and mechanical ventilation (29), but Papenburg et al. (30) reported that there was no significant difference in the severity of the two.
Our study has better objectivity and fewer data errors due to the large sample size and better representation due to the long study period. However, there were several limitations in our study. First, we only analyzed the epidemiological characteristics of RSV infection in hospitalized children with CAP in Guangzhou. Second, limited by the sample collection protocol, we were unable to acquire detailed information on coinfection in RSV-negative children, and potential coinfection with bacteria or other viruses in the non-RSV group may bias the results. In addition, we did not test the sputum, and possible pathogens in sputum were not available in our study. Although previous studies have demonstrated that the sensitivity and specificity of oropharyngeal swabs are relatively high (31), the detected pathogens may not be the cause of pneumonia (32). Thus, the correlation between RSV and severe CAP cannot be confidently stated, as our study did not analyze other pathogens, such as bacteria that cause pneumonia.
Conclusions
In this study, the data on RSV infection in hospitalized children with CAP in Guangzhou from 2010 to 2019 were analyzed in many respects. The current study suggested that January to March were the key months for prevention and control of CAP caused by RSV in Guangzhou, and children less than 0.5 years old were the key population for prevention and control of CAP caused by RSV. Policy makers should make timely adjustments to prevention measures and medical resources based on these epidemiological characteristics. Our study also found that RSV-positive CAP children were more likely to develop severe pneumonia, which suggested the importance of early treatment and close attention in these patients’ and CT values of RSV may be helpful in assessing the risk of severe pneumonia.
Acknowledgments
Funding: This project was funded by the National Natural Science Foundation of China (No. 81970003); Guangzhou Science and Technology Program-Zhongnanshan Medical Foundation of Guangdong Province (No. 202102010359-ZNSA-2020003); Guangdong-Hong Kong-Macao Joint Laboratory of Respiratory Infectious Diseases Subject (No. GHMJLRID-Z-202109) and Special Project for COVID-19 Prevention and Control of Zhongnanshan Medical Foundation of Guangdong Province (No. ZNSA2020012).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-331/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-331/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-331/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-331/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First Affiliated Hospital of Guangzhou Medical University Ethics Committee (No. 20170315), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong-Chew RM, García-León ML, Noyola DE, et al. Respiratory viruses detected in Mexican children younger than 5 years old with community-acquired pneumonia: a national multicenter study. Int J Infect Dis 2017;62:32-8. [Crossref] [PubMed]
- Yu Y, Fei A. Atypical pathogen infection in community-acquired pneumonia. Biosci Trends 2016;10:7-13. [Crossref] [PubMed]
- Global Burden of Disease Pediatrics Collaboration. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr 2016;170:267-87. [Crossref] [PubMed]
- Resch B. Burden of respiratory syncytial virus infection in young children. World J Clin Pediatr 2012;1:8-12. [Crossref] [PubMed]
- Subspecialty Group of Respiratory Diseases, The Society of Pediatrics; Chinese Medical Association The Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of community acquired pneumonia in children(the revised edition of 2013) (II). Zhonghua Er Ke Za Zhi 2013;51:856-62.
- Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I). Zhonghua Er Ke Za Zhi 2013;51:745-52.
- Liu WK, Liu Q, Chen DH, et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis 2013;13:28. [Crossref] [PubMed]
- Liu WK, Chen DH, Liu Q, et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis 2011;11:345. [Crossref] [PubMed]
- Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835-45. [Crossref] [PubMed]
- Carvajal JJ, Avellaneda AM, Salazar-Ardiles C, et al. Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front Immunol 2019;10:2152. [Crossref] [PubMed]
- Tsafaras GP, Ntontsi P, Xanthou G. Advantages and Limitations of the Neonatal Immune System. Front Pediatr 2020;8:5. [Crossref] [PubMed]
- van den Berg JP, Westerbeek EA, van der Klis FR, et al. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev 2011;87:67-72. [Crossref] [PubMed]
- Gans H, DeHovitz R, Forghani B, et al. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine 2003;21:3398-405. [Crossref] [PubMed]
- Radhakrishnan D, Ouedraogo A, Shariff SZ, et al. The association between climate, geography and respiratory syncitial virus hospitalizations among children in Ontario, Canada: a population-based study. BMC Infect Dis 2020;20:157. [Crossref] [PubMed]
- Uekert SJ, Akan G, Evans MD, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol 2006;118:1375-81. [Crossref] [PubMed]
- Guan X, Polesso F, Wang C, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022;606:791-6. [Crossref] [PubMed]
- Weinstein Y, Berkovich Z. Testosterone effect on bone marrow, thymus, and suppressor T cells in the (NZB X NZW)F1 mice: its relevance to autoimmunity. J Immunol 1981;126:998-1002. [Crossref] [PubMed]
- Yu J, Xie Z, Zhang T, et al. Comparison of the prevalence of respiratory viruses in patients with acute respiratory infections at different hospital settings in North China, 2012-2015. BMC Infect Dis 2018;18:72. [Crossref] [PubMed]
- Le Geyt J, Hauck S, Lee M, et al. Respiratory syncytial virus prevalence in children admitted to five Kenyan district hospitals: a cross-sectional study. BMJ Paediatr Open 2019;3:e000409. [Crossref] [PubMed]
- Liu W, Chen D, Tan W, et al. Epidemiology and Clinical Presentations of Respiratory Syncytial Virus Subgroups A and B Detected with Multiplex Real-Time PCR. PLoS One 2016;11:e0165108. [Crossref] [PubMed]
- Cui G, Zhu R, Qian Y, et al. Genetic variation in attachment glycoprotein genes of human respiratory syncytial virus subgroups a and B in children in recent five consecutive years. PLoS One 2013;8:e75020. [Crossref] [PubMed]
- Zhang H, Wen S, Zheng J, et al. Meteorological factors affecting respiratory syncytial virus infection: A time-series analysis. Pediatr Pulmonol 2020;55:713-8. [Crossref] [PubMed]
- Song J, Zhang Y, Wang H, et al. Emergence of ON1 genotype of human respiratory syncytial virus subgroup A in China between 2011 and 2015. Sci Rep 2017;7:5501. [Crossref] [PubMed]
- Ning G, Wang X, Wu D, et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001-2015: A systematic review. Hum Vaccin Immunother 2017;13:2742-50. [Crossref] [PubMed]
- Liu P, Xu M, He L, et al. Epidemiology of Respiratory Pathogens in Children with Lower Respiratory Tract Infections in Shanghai, China, from 2013 to 2015. Jpn J Infect Dis 2018;71:39-44. [Crossref] [PubMed]
- Munywoki PK, Ohuma EO, Ngama M, et al. Severe lower respiratory tract infection in early infancy and pneumonia hospitalizations among children, Kenya. Emerg Infect Dis 2013;19:223-9. [Crossref] [PubMed]
- Sun YP, Zheng XY, Zhang HX, et al. Epidemiology of Respiratory Pathogens Among Children Hospitalized for Pneumonia in Xiamen: A Retrospective Study. Infect Dis Ther 2021;10:1567-78. [Crossref] [PubMed]
- Rossi GA, Colin AA. Respiratory syncytial virus-Host interaction in the pathogenesis of bronchiolitis and its impact on respiratory morbidity in later life. Pediatr Allergy Immunol 2017;28:320-31. [Crossref] [PubMed]
- Youssef Y, Chmaisse A, Boutros C, et al. The burden of Respiratory Syncytial Virus (RSV) infection in the Middle East and North Africa (MENA) region across age groups: A systematic review. Vaccine 2021;39:3803-13. [Crossref] [PubMed]
- Papenburg J, Hamelin MÈ, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis 2012;206:178-89. [Crossref] [PubMed]
- Wang L, Yang S, Yan X, et al. Comparing the yield of oropharyngeal swabs and sputum for detection of 11 common pathogens in hospitalized children with lower respiratory tract infection. Virol J 2019;16:84. [Crossref] [PubMed]
- Mardian Y, Menur Naysilla A, Lokida D, et al. Approach to Identifying Causative Pathogens of Community-Acquired Pneumonia in Children Using Culture, Molecular, and Serology Tests. Front Pediatr 2021;9:629318. [Crossref] [PubMed]


