
Techniques and outcomes of percutaneous aortic anastomosis leak closure after frozen elephant trunk procedure for aortic dissection
Highlight box
Key findings
• Percutaneous closure of aortic anastomosis leak (AAL) after frozen elephant trunk (FET) procedure could be performed safely with a high success rate and a low incidence of complications.
What is known and what is new?
• AAL after FET causes false lumen patency and a poor prognosis of aortic dissection.
• The magnitude of benefit is greatest with AAL reduction to mild or minimal, which the false lumen of aortic dissection decreases quickly.
What is the implication, and what should change now?
• Percutaneous closure is an alternative therapy for patients with AAL after FET procedure.
Introduction
The frozen elephant trunk (FET) technique is an effective surgical repair for acute and chronic aortic dissections (1-4). The covering of proximal tears in the descending aorta through an FET stent-graft induces false lumen thrombosis and promotes satisfactory aortic remodeling (5). However, proximal aortic anastomosis leak (AAL), which is defined as a shunt originating from the aortic lumen through the suture dehiscence between the FET stent-graft and the native aorta, occurs in 4.3% patients after the FET procedure (6,7). This complication causes false lumen patency and a poor prognosis of aortic dissection, which should be corrected early. Surgical repair is a standard therapy for AAL but is associated with high morbidity and mortality (8,9). Therefore, percutaneous AAL repair has emerged as an alternative therapy for patients with AAL. A few smaller studies have reported different endovascular strategies for AAL lesions (10-13).
Percutaneous AAL closures using various devices have been attempted at our center. We assumed that AAL closures could be achieved with high success rates and would improve the clinical outcomes of the aortic dissection (14). In this study, we report our techniques and outcomes of percutaneous closures of AALs in patients who underwent FET procedures for aortic dissections. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1092/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was performed with the approval of Beijing Anzhen Hospital Institutional Review Board (approval No. 2022216X). Informed consent was obtained from all patients included in the study. Records were analyzed for each patient that underwent percutaneous AAL closure after an FET procedure for aortic dissection between January 2016 and December 2020. The indication for an AAL closure was the leak inducing false lumen patency of aortic dissection after the primary FET procedure, which was identified based on an aortic computed tomography angiography (CTA).
Percutaneous AAL closure technique
High-quality aortic CTA played a crucial role in preparing to perform an AAL closure. The exact anatomic conditions—such as the shape, size, number, and precise location of the aortic leak—should be identified clearly. Furthermore, distal intimal tears in the descending aorta, the abdominal aorta, and the iliac artery should be evaluated during a pre-procedure assessment. This step was necessary for introducing the delivery system through the femoral artery. Adequate planning shortened procedure time and improved the success rates of closures.
Three percutaneous techniques can be used in AAL closures: (I) the retrograde technique; (II) the true-to-false lumen loop technique; and (III) the antegrade technique (Figure 1). The retrograde technique was the most common approach for percutaneous AAL closures at our center. This method was expeditious for a majority of AAL closures when crossing the leak smoothly. First, a 6 French access was placed in the femoral artery. However, because the vast majority of aortic dissections did not involve the femoral artery, a suitable distal intimal tear in the descending aorta, the abdominal aorta, or iliac artery was crossed with a 260-cm 0.035-inch hydrophilic wire (Terumo, Tokyo, Japan) through a Judkins right 4.0 catheter or a Cobra catheter. The catheter was then inserted in the FET AAL defect after it was advanced from the aortic true lumen into the false lumen. After replacing the glide wire with an Amplatzer super stiff guidewire (Boston Scientific, Marlborough, MA, USA), a compatible delivery sheath was introduced. A proper Amplatzer device was then deployed across the leak anterogradely. A device 0 to 2 mm larger than the defect diameter was usually selected depending on the CTA measurement. Various devices from Amplatzer family, such as the Amplatzer duct occluder (ADO) I (Lifetech, Shenzhen, China), ADO II (Abott, Santa Clara, CA, USA), ventricular septal occluder (VSO) (Lifetech, Shenzhen, China), and Amplatzer vascular plug (AVP) (Abott), were used in the study.
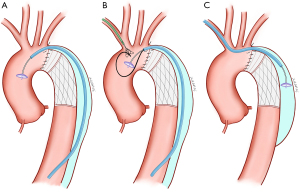
In extremely calcified or tortuous leaks, additional support might be required to facilitate wire crossing and the delivery of equipment. In such cases, a true-to-false lumen loop can assist catheter and device delivery systems. The guide wire used to cross the leak was advanced into the ascending aorta and then snared and exteriorized to the brachial artery. The delivery sheath was an upsized 1 French to accommodate the occlusion device alongside the loop wire, and the occluder was deployed across the defect next to the guidewire. The remaining loop allowed access to the AAL to be maintained during device deployment attempts.
The antegrade technique is only used where there is no distal intimal tear for advancing the delivery system into the aortic false lumen. Through this approach, right brachial artery access can be achieved. The delivery sheath was advanced through the aortic true lumen into the false lumen, and then the occlusion device was implanted. A disadvantage of this technique was that device choices were limited. Firstly, the front disk of Amplatzer occluder was placed in the false lumen (the low-pressure area), which meant that a symmetric device was more suitable for closing the AAL. Subsequently, the main body of the device was placed in the aortic arch. If the device was pulled too deep, interference with the aortic branch blood flow might occur after deployment or release of the device. An aortogram was required to verify the correct positioning of the device. Additionally, the complication rate of brachial artery access was higher than femoral artery access.
Statistical analysis
Continuous variables are described as mean ± standard deviation (SD) or median (interquartile range) and compared using t-tests. All analyses were performed with SPSS version 20 (IBM, Armonk, NY, USA). Statistical significance was inferred at P<0.05.
Results
Between December 2016 and January 2020, 34 AAL closure procedures were performed in 32 patients. Two patients underwent a secondary percutaneous closure because of a recurrence leak. The mean age of the study population was 44.3±9.1 years, and 28 patients (87.5%) were male. The indications for primary aortic surgery were Stanford A dissections in 24 patients (75%) and complex Stanford B dissections in 8 patients (25%). Our team utilized various strategies for ascending aorta and aortic arch combined FET procedures in the primary surgery: 13 patients (40.6%) had an ascending aorta replacement, 10 patients (31.3%) received aortic valve combined with ascending aorta replacement, 4 patients (12.5%) received a transposition of arch branches, and 5 patients (15.6%) did not receive an intervention for ascending aorta or arch. Three patients had Marfan syndrome, which was inappropriate for thoracic endovascular aortic repair (TEVAR). One patient had chronic dissecting aneurysm, and a planned second-stage thoracoabdominal aortic replacement will be performed in the future. Another had an acute type B dissection with a large intimal tear and inadequate landing zone. Therefore, TEVAR was not performed in these 5 patients.
Of note, most patients in this series (n=25, 78.1%) had no symptoms, and the AALs were detected during routine follow-up. Of the remaining 7 patients, 5 experienced dyspnea (15.6%), and 2 patients had chest pain (6.3%). The primary indication for closure of AAL was persistent perfusion of the false lumen at the FET level. The mean time from FET procedure to percutaneous closure was 28.3±28.1 months. The baseline characteristics of patients are shown in Table 1.
Table 1
| Baseline characteristics | Number (%) or mean ± SD |
|---|---|
| Age (years) | 44.3±9.1 |
| Male | 28 (87.5) |
| Hypertension | 32 (100.0) |
| Hyperlipidemia | 4 (12.5) |
| Marfan syndrome | 4 (12.5) |
| Diabetes | 3 (9.3) |
| Coronary artery disease | 6 (18.8) |
| Renal insufficiency | 5 (15.6) |
| Chronic obstructive pulmonary disease | 1 (3.1) |
| Stroke | 2 (6.3) |
| Classifications of aortic dissection | |
| Stanford A | 24 (75.0) |
| Stanford B | 8 (25.0) |
| Strategies for ascending aorta and aortic arch | |
| Bentall procedure | 10 (31.3) |
| Ascending aorta replacement | 13 (40.6) |
| Transposition of arch branches | 4 (12.5) |
| No intervention | 5 (15.6) |
| Prior FET procedure time (months) | 75.4±44.7 |
| FET-to-closure time (months) | 28.3±28.1 |
| Symptoms of leak | |
| Chest pain | 2 (6.3) |
| Dyspnea | 5 (15.6) |
| No symptoms | 25 (78.1) |
FET, frozen elephant trunk; SD, standard deviation.
Technical outcomes
Both successful leakage crossing and device deployment were achieved in all 32 patients. A total of 36 occlusion devices were deployed in 36 leaks. The ADO I was used in most cases (n=16, 44.4%), followed by VSO (n=8, 22.1%), ADO II (n=6, 16.7%), AVP III (n=3, 8.3%), AVP IV (n=2, 5.6%), and AVP II (n=1, 2.8%). The most common AAL closure technique was the retrograde approach (n=27, 79.4%). The true-to-false lumen loop technique and the antegrade approach were used in 6 procedures (17.6%) and 1 procedure (2.9%), respectively. Technical success—defined as successful device implantation associated with a mild or decreased residual leak—was achieved in 29 patients (90.6%). The immediate residual leaks were moderate in 3 patients (9.4%). An in-hospital, major adverse event occurred in 1 patient (3.1%). The patient experienced a femoral artery pseudoaneurysm, which was treated by surgical repair. The mean length of hospital stay was 2.3±1.2 days (Table 2).
Table 2
| Technique and results | Number (%) or mean ± SD |
|---|---|
| Patients | 32 |
| Procedures | 34 |
| AALs | 36 |
| Follow-up time (months) | 47.1±24.6 |
| Distance between AAL and proximal branch (mm) | 2.8±2.5 |
| Technical strategies | |
| Retrograde technique | 27 (79.4) |
| True-to-false lumen loop technique | 6 (17.6) |
| Antegrade technique | 1 (2.9) |
| Number of devices placed | |
| 1 | 28 (87.5) |
| 2 | 4 (12.5) |
| Device types | |
| ADO I | 16 (44.4) |
| VSO | 8 (22.1) |
| ADO II | 6 (16.7) |
| AVP III | 3 (8.3) |
| AVP IV | 2 (5.6) |
| AVP II | 1 (2.8) |
| Procedure time (min) | 33±17 |
| Fluoroscopy time (min) | 14±7 |
| Immediate residual shunt | |
| None or trivial | 17 (53.1) |
| Mild | 12 (37.5) |
| Moderate or more | 3 (9.4) |
| Follow-up residual shunt | |
| None or trivial | 24 (75.0) |
| Mild | 5 (15.6) |
| Moderate or more | 3 (9.4) |
| Degrees of false lumen thrombosis | |
| Complete | 24 (75.0) |
| Basically complete | 5 (15.6) |
| Partial | 3 (9.4) |
| Pre-procedure diameter of false lumen (mm) | 33.0±9.4 |
| Post-procedure diameter of false lumen (mm) | 19.4±16.2 |
| Length of stay (days) | 2.3±1.2 |
AAL, aortic anastomosis leak; ADO, Amplatzer duct occluder; VSO, ventricular septal occluder; AVP, Amplatzer vascular plug; SD, standard deviation.
Follow-up outcomes
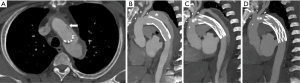
The mean time to follow-up was 47.1±24.6 months. Among the 32 patients, 1 death occurred 2 months after successful AAL closure due to a catastrophic hemorrhage during a redo aortic valve replacement. The moderate residual leak in the 3 patients did not change during follow-up. However, of the 12 patients with a mild residual leak, only 5 patients had a persistent, mild residual leak. Varying degrees of thrombosis of the FET’s segment false lumen were achieved in all 32 patients. Twenty-four patients (75%) had complete thrombosis of the FET’s segment false lumen, 5 patients (15.6%) had a near complete thrombosis of the FET’s segment false lumen, and 3 (9.4%) had only partial thrombosis of the FET’s segment false lumen. The 3 patients that had partial thrombosis were the patients who had a moderate residual shunt after the AAL closure. The primary measure of efficacy of the AAL closure was the diameter of the aorta. The maximal diameter of FET’s segment false lumen significantly decreased 13.6±8.7 mm (from 33.0±9.4 to 19.4±16.2 mm, P<0.001) 1 year after AAL closure (Table 2). Figure 2 shows the CTA in preprocedural planning and follow-up results after closure. It is noteworthy that the false lumen did not change in the 3 patients with a moderate residual leak. One patient underwent a successful surgical repair, but the other 2 patients refused additional surgical interventions because of pain from prior procedures.
Discussion
The principle findings of our study are as follows: (I) percutaneous closure of AAL after FET may be performed safely with a high success rate of device deployment and a low incidence of procedure related complications; (II) occluder deployment was achieved in all study patients, and there was a noteworthy reduction of the AAL to mild or minimal after a successful closure in a majority of patients (90.6%); and (III) successful AAL reduction to mild or minimal resulted in quick false lumen decreases and reduced the need for redo aortic surgery.
The FET procedure has grown in popularity as an effective surgical approach for acute and chronic aortic dissection. Aortic shape augmentation is due to growth of the false lumen after the FET procedure. Nevertheless, the proximal AAL creates direct blood flow to the false lumen, thereby preventing false lumen thrombosis. False lumen patency is described as an independent risk factor for subsequent interventions because it leads to malperfusion syndrome, aneurysm, and thus, aortic rupture (15,16). However, most patients with a proximal AAL after a FET procedure have no symptoms, and routine follow-up is essential for these patients. The AAL should be treated once it is detected, although the risk for aortic redo surgery after the FET procedure remains substantial (17).
A number of endovascular strategies that provide minimally invasive options to patients who may not be candidates for redo surgical procedures have been reported. Endovascular repair using a stent-graft is not widely used due to the often insufficient landing zone (18), and the stent-graft might occlude branch arteries, including the coronary arteries and arch arteries. While coil embolization has been reported as an effective therapy in some cases, device migration is likely to occur in patients with high-speed flow (19). Several recent reports have also suggested the utility of the Amplatzer devices for treating AAL (20,21). We have also reported our experience with percutaneous closures using Amplatzer devices in different classifications of AAL (12). However, the methods and efficacy of percutaneous closure in AAL after the FET procedure had not yet been reported.
In this study, we described 3 different techniques for percutaneous AAL closures after an FET procedure for aortic dissection. Important technical considerations should be taken into account to maximize efficiency of and minimize risks associated with the procedure. In addition, knowledge of device requirements (e.g., diameter, length, and sheath-size) may reduce the duration and cost of the procedure. Of the 3 procedures, the retrograde technique, which can be completed rapidly and easily, was our preferred approach. Preoperative CTA evaluation was used to select a distal intimal tear with an appropriate location and size, which was then used to insert the device into the false lumen. The true-to-false lumen loop technique was used in some cases to provide a more stable rail for crossing the leak. On rare occasions, percutaneous AAL closures using the antegrade approach may lead to aortic arch branch obstruction. Aortography and selective angiography may be needed to assess ostial clearance before device release. Meticulous cardiac computed tomographic measurement and analyses can identify the relationship between the AAL and supra-aortic trunks before the procedure. A sufficient rim of tissue must exist around the leak for coaxial apposition of the occluder with the aorta to prevent device migration. It is also important to ensure adequate distance of the AAL from the arch branch arteries and measure the closure device accordingly. Rarely, device interference can occur after occluder release due to tilting of the device, which would require its removal with a snare.
As illustrated above, successful crossing of AALs and deployment of occluders was achieved in all patients; however, elimination of AAL residual shunts was still technically difficult. Residual shunts after device releasing during the procedures were detected in nearly half of patients. This can be due to several reasons. The AAL is a suture tear of the prosthesis without tissue elasticity, which can make complete abolition of the leak more challenging. Despite using various Amplatzer occluders in our study, there are no devices designed specifically for AAL closures. Many patients have irregular shaped leaks, and the device may not fit the defect. Based on our experiences, increasing the size of the occluder may not reduce the residual shunt, and excessive radial force from the device may interfere with the aortic prosthesis. Where possible, we do not oversize the occlusion device, and if we do, it is only by 2 mm. The follow-up results showed that the residual shunt would reduce after device endothelialization. However, patients in our study with a moderate residual shunt did not show a change. If the false lumen is markedly enlarged during follow-up, further interventions should be performed. Complete or near complete obliteration of AAL was accomplished in the majority (90.6%) of patients following the procedure.
In our study, 2 patients needed reintervention after a successful closure. We hypothesized 2 possible reasons for a recurrence of AAL: (I) a very small leak might be ignored if a larger leak nearby has not been closed; or (II) conversely, a small AAL could worsen during extended follow-up. We believe a rigorous CTA follow-up after both the FET procedure and percutaneous AAL closure is required.
Study limitation
The retrospective study design is associated with inherent limitations. Our series involved a single center-experience without a control group of AAL redo surgical repair, and it covered a long period of time during which our understanding and management of AAL significantly improved. In addition, due to the limited numbers of procedures, we could not determine if a particular type of Amplatzer device facilitated better outcomes in our study population.
Conclusions
Percutaneous closure of AAL after an FET procedure could be performed with high technical success and low complication rates. Successful AAL closures resulted in quick aortic false lumen diameter reduction and decreased the need for redo aortic surgery. This technique appeared to be a fast, safe, cost-sparing, and efficient therapy for patients who would face multiple risks with a surgical intervention.
Acknowledgments
We would like to thank Dr. Kelsey Marx for her help in polishing our paper. We also appreciate Dr. Xuan Fang for drawing the figures in our paper.
Funding: This study was supported by the Beijing Municipal Administration of Hospitals Incubating Program (Code: PX2019024).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1092/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1092/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1092/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1092/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was performed with the approval of Beijing Anzhen Hospital Institutional Review Board (approval No. 2022216X). Informed consent was obtained from all patients included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tian DH, Ha H, Joshi Y, et al. Long-term outcomes of the frozen elephant trunk procedure: a systematic review. Ann Cardiothorac Surg 2020;9:144-51. [Crossref] [PubMed]
- Luo C, Qi R, Zhong Y, et al. Early and Long-Term Follow-Up for Chronic Type B and Type Non-A Non-B Aortic Dissection Using the Frozen Elephant Trunk Technique. Front Cardiovasc Med 2021;8:714638. [Crossref] [PubMed]
- Yoshitake A, Tochii M, Tokunaga C, et al. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg 2020;58:707-13. [Crossref] [PubMed]
- Qiao Z, Chen S, Guo R, et al. Comparison of Open Repair vs. the One-Stage Hybrid Extra-Anatomic Technique for Distal Aortic Arch Disease Treatment: Mid-term Outcomes With a Risk-Adjusted Analysis. Front Cardiovasc Med 2021;8:725902. [Crossref] [PubMed]
- Kozlov BN, Panfilov DS. False lumen thrombosis after frozen elephant trunk procedure in acute and chronic aortic dissection. J Cardiovasc Surg (Torino) 2022;63:195-201. [Crossref] [PubMed]
- Hiraoka A, Chikazawa G, Sakaguchi T, et al. Late leakage from four-branch prosthetic graft after total aortic arch repair. Eur J Cardiothorac Surg 2016;49:e31-2. [Crossref] [PubMed]
- Ghazy T, Mahlmann A, Fajfrova Z, et al. Anastomotic leak after surgical repair of type A aortic dissection - prevalence and consequences in midterm follow-up. Vasa 2017;46:377-82. [Crossref] [PubMed]
- Charchyan E, Breshenkov D, Belov Y. Follow-up outcomes after the frozen elephant trunk technique in chronic type B dissection. Eur J Cardiothorac Surg 2020;57:904-11. [Crossref] [PubMed]
- Kreibich M, Berger T, Rylski B, et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg 2020;159:392-399.e1. [Crossref] [PubMed]
- Falkenberg M, Roos H, Lepore V, et al. Endovascular Closure of Chronic Dissection Entries in the Aortic Arch Using the Amplatzer Vascular Plug II as a Sealing Button. J Endovasc Ther 2016;23:378-83. [Crossref] [PubMed]
- Tomasi J, Belhaj Soulami R, Rolland M, et al. Endovascular Repair of a Dacron Pseudoaneurysm in an Ascending-to-Descending Aortic Bypass. Aorta (Stamford) 2020;8:104-6. [Crossref] [PubMed]
- Pu J, Ke Y, Huang L, et al. Transcatheter Closure of Aortic Anastomosis Leak Resulting in Patent Cabrol Shunt After Aortic Replacement. JACC Cardiovasc Interv 2017;10:2126-8. [Crossref] [PubMed]
- Pajtak R, Lovelock T, Nanayakkara S, et al. Avoiding the re-do sternotomy: treatment of an ascending aortic pseudoaneurysm using a percutaneous Amplatzer PFO closure device. Vascular 2022; Epub ahead of print. [Crossref] [PubMed]
- Wu W, Ke Y, Zhao H, et al. Trans-catheter closure of aortic anastomosis leak after aortic replacement: classifications and techniques. J Thorac Dis 2020;12:4883-91. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [Crossref] [PubMed]
- Czerny M, Barchichat I, Meszaros K, et al. Long-term results after proximal thoracic aortic redo surgery. PLoS One 2013;8:e57713. [Crossref] [PubMed]
- Arima D, Suematsu Y, Nishi S, et al. Zone 0 Thoracic Endovascular Aortic Repair Using Reverse Extra-Anatomical Aortic Arch Debranching Technique for an Anastomotic Pseudoaneurysm and Acute Aortic Dissection that Developed after Bentall's Surgery Combined with Sjögren's Syndrome. Ann Vasc Dis 2020;13:103-6. [Crossref] [PubMed]
- Sodian R, Schmauss D, Schmitz C, et al. 3-dimensional printing of models to create custom-made devices for coil embolization of an anastomotic leak after aortic arch replacement. Ann Thorac Surg 2009;88:974-8. [Crossref] [PubMed]
- Pu J, Huang L, Wu W. Transcatheter Closure of Anastomotic Leakage After Aortic Surgery for Type A Dissection with the Amplatzer Duct Occluder II. Cardiovasc Intervent Radiol 2017;40:1274-7. [Crossref] [PubMed]
- Sulemankhil I, Topalidis D, Verma A, et al. Closure of a Thoracic Aortic Graft Pseudoaneurysm With an Amplatzer Septal Occluder. Ann Thorac Surg 2021;112:e13-5. [Crossref] [PubMed]


