
Utilizing computed tomography volumetry for size matching prior to lung transplantation: a case series
Highlight box
Key findings
• In four cases, CT volumetry facilitated the confident acceptance of donor lungs that were initially predicted to be oversized based on other clinical measures.
What is known and what is new?
• Appropriate size matching between donor and recipient is crucial for successful pulmonary transplantation, but current methods of estimating lung volumes vary widely and have poor predictive value.
• CT volumetry has been reported as an effective method for estimating lung volume.
What is the implication, and what should change now?
• CT volumetry is a valuable an adjunct for decision-making regarding the suitability of donor lungs for transplantation, and further investigation is warranted to determine potential impact on clinical outcomes.
Introduction
Accurate size matching between donor lungs and a recipient’s thoracic cavity is a crucial consideration in thoracic transplantation and an important determinant in clinical outcomes. Inappropriately sized donor lungs increase the surgical complexity of implantation, and large discrepancies in the size of airway or vascular lumens can increase the risk of anastomotic complications. Oversized lung grafts make the operation technically more challenging due to difficult hilar exposure and often require downsizing with a lobectomy or sublobar resection. However, retrospective analyses using predicted lung capacity ratios have demonstrated that oversized lung allografts are associated with decreased odds of primary graft dysfunction, bronchiolitis obliterans syndrome, and improved long-term survival (1-3). Conversely, undersized lung grafts are associated with an increased occurrence of primary graft dysfunction, longer hospitalizations, and increased risk of tracheostomy and airway complications (1). Importantly, size considerations limit organ availability and have a significant impact on waitlist mortality, as evidenced by patient height being a leading independent predictor for survival in patients awaiting lung transplantation (LT) (4). Thus, refined strategies for accurate size matching between donors and recipients are needed to optimize organ allocation and improve clinical and functional outcomes after transplantation.
Several methods have been utilized to estimate donor and recipient lung sizes, including submammary thoracic perimeter, thoracic height and diameter on chest X-ray (CXR), patient height, and weight (5,6). Although true total lung capacity (TLC) values measured by plethysmography provide accurate representations of lung volume, this testing typically cannot be obtained in critically ill organ donors (7). Alternatively, calculating an individual’s predicted TLC (pTLC) based on age, height, and gender has been proposed as an objective measure (7-9). However, pTLC is not commonly utilized in clinical practice due to its failure to incorporate many other salient patient factors that influence chest cavity size, such as weight, race, and etiology of the recipient’s pulmonary disease. While calculating pTLC estimates the maximum volume of gas that can be drawn into a pair of lungs, effective size matching in transplantation involves anatomical comparison between the volume of donor lungs and available space within the recipient’s thoracic cavity. Thus, lung volume measurements using computed tomography (CT) volumetry, which are generated from automated identification of voxels representing lung parenchyma and the surrounding chest wall and translated into quantified lung volumes, serve as an accurate representation of true lung size for determining donor suitability (10).
CT volumetry has been reported as an effective method for estimating lung volume in the setting of thoracic transplantation (11-13). Furthermore, CT volumetry has been shown to be a useful adjunct for determining donor-recipient size matching of individual lung lobes in living-donor lobar LT and unilateral LT (14,15). However, prior reports have not described the prospective use of pre-operative CT volumetry as an adjunct for decision-making regarding size matching in bilateral LT.
Here, we describe our initial experience utilizing CT volumetry for donor and recipient lung volume assessment and discuss the limitations and implications of its use in clinical practice. We performed a single center exploratory study of four LT cases in which both the recipient and donor underwent CT volumetry pre-operatively to aid with decision-making regarding organ size and suitability. The donor-recipient dyads were selected for volumetric analysis due to uncertainty about size matching based upon conventional criteria including height and CXR lung heights. Data were collected from our institutional transplant recipient database and from the electronic medical records. We reviewed patient-specific factors including sex, age, height, body mass index (BMI), lung heights on CXR, indication for LT, and most recent pulmonary function testing values. Recipient CT exams were performed on a dual source multidetector Siemens SOMATOM Force 384-slice scanner (model number 10742326, Siemens Medical, Billerica, MA, USA) with software version VB20 (SP4) during deep inspiratory breath hold. Donor CT scans were performed during continuous mechanical ventilation without breath hold. Non-contrast transaxial images of the chest were obtained in the supine position with arms extended overhead according to a high-resolution protocol with at least 0.4 mm slice thickness and a 1.5-mm reconstruction interval. CT volumetry was performed by a board-certified radiologist (author AJB) using commercially available software (Vitrea; Vital Images Inc., Minnetonka, MN, USA) on an independent workstation (Extended Brilliance Workspace; Phillips Medical Systems). This software generates automated segmentation of lungs based on a threshold density of −750 Hounsfield unit (HU) and region of interest, quantifying three-dimensional volume-rendered images that automatically calculate lung volume (CTvol) as the sum of both lung volumes of voxels from segmentation. Postoperative CT volumetry was not obtained, as repeat CT imaging is not routinely performed in recipients following LT per our institutional protocols. pTLC was calculated utilizing the standardized formulas proposed by the European Respiratory Society (9). We present the following article in accordance with the AME Case Series reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1203/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent according to the Washington University School of Medicine institutional review board.
Case 1
A 39-year-old female with pulmonary arterial hypertension was listed for bilateral LT. A suitable donor was identified as a 37-year-old male who was taller than the recipient (1.8 vs. 1.7 m) with a similar BMI (33 vs. 34 kg/m2). Lung heights measured on CXR were comparable between the donor and recipient [right (R) 19/left (L) 21 vs. R 21/L 23 cm]. However, the calculated pTLC of the donor was markedly higher than that of the recipient (7,329 vs. 5,529 mL). CT volumetric analysis was performed, which demonstrated nearly equivalent CTvol between the donor and recipient (4,937 vs. 5,275 mL). Therefore, the decision was made to proceed with transplantation. The patient underwent successful LT performed on veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) and the grafts did not require downsizing. Her remaining post-operative course was uncomplicated and she was discharged home on post-operative day 13 without supplemental oxygen.
Case 2
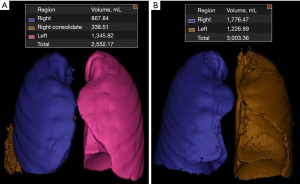
A 20-year-old female with pulmonary arterial hypertension was listed for bilateral LT and received a donor offer from a 15-year-old male who was taller (1.8 vs. 1.6 m) but with smaller lung heights on CXR (R 22/L 19 vs. R 23/L 24 cm). The donor’s pTLC was much larger than the recipient’s (7,329 vs. 4,770 mL). CT volumetry data was obtained which revealed similar donor and recipient CTvol (2,552 vs. 3,003 mL, respectively) (Figure 1). The figure demonstrates mild consolidation/atelectasis in the donor right lower lobe, likely contributing to some underestimation of the lung volume. With these data, the decision was made to proceed with transplantation. The procedure was performed on VA ECMO and grafts did not require downsizing. Post-operatively, the patient had an uncomplicated course and was discharged home on post-operative day 13 without supplemental oxygen.
Case 3
A 65-year-old female with interstitial lung disease was listed for bilateral LT. A potential donor was identified as a 54-year-old female who appeared smaller by multiple metrics (BMI 22 vs. 30 kg/m2; height 1.6 vs. 1.7 m; pTLC of 4,771 vs. 5,272 mL). However, the donor had taller lung heights on CXR than the recipient (R 25/L 23 vs. R 19/L 22 cm). CT volumetry was performed which demonstrated that both the donor and recipient had much smaller CTvol (donor 2,858 mL vs. recipient 1,917 mL) than estimated by the pTLC. CT volumetry of the individual donor lung volumes (R 1,528 mL and L 1,330 mL) and recipient lung volumes (R 1,115 mL and L 802 mL) showed that the CTvol discrepancy was primarily localized on the left side, due to the higher burden of fibrotic lung disease in the left lung which was also shown on the patient’s ventilation-perfusion scintigraphy. Considering these data points collectively, the donor was deemed an acceptable match to provide mildly oversized grafts. In this particular recipient, we were prepared to perform a back table downsizing including a lobectomy of the left lung. However, intra-operative findings demonstrated that the graft was only mildly oversized for the left pleural space. Our experience has been that a delayed chest closure often mitigates the need for significant downsizing, as was performed in this case. Transplantation was performed on VA ECMO and following delayed chest closure, the patient had a prolonged post-operative hospital course requiring tracheostomy and gradual weaning from mechanical ventilation. She was discharged on post-operative day 42 without supplemental oxygen.
Case 4
A 61-year-old female with chronic obstructive pulmonary disease (COPD) was listed for bilateral LT. A potential donor was identified a 61-year-old male who appeared larger by clinical metrics (BMI 35 vs. 21 kg/m2; height 1.9 vs. 1.7 m), with markedly oversized lungs based on pTLC volumes (8,141 vs. 5,100 mL). CT volumetry was performed, which demonstrated that the donor lungs were actually smaller than the recipient’s (3,396 and 6,014 mL). Thus, the donor lungs were accepted. The patient underwent successful transplantation without complication and was discharged home on post-operative day 17 without supplemental oxygen.
Discussion
In these four cases, CT volumetry facilitated confidently accepting donor lungs that initially appeared poorly size matched based on pTLC and other conventional clinical parameters. Importantly, large differences were observed between pTLC volumes and CT volumetry in all four cases, thus underscoring the inaccuracy of relying on pTLC values (which are calculated using generic formulas which only incorporate age and height with slight adjustments for gender). These formulas fail to incorporate important factors such as weight, thoracic cavity parameters, race, and etiology of pulmonary disease. Additionally, the smaller lung volumes measured by CT volumetry may reflect clinical factors such as atelectasis, consolidation, body habitus, or continuous breathing during donor CT scan acquisition. While other formulas have been proposed to incorporate additional patient factors, their practicality remains limited by cumbersome calculations (11). In this sense, CT volumetry offers a more practical and dynamic method for assessing donor and recipient lung sizes, especially in patients with hyperinflated lungs or restrictive lung disease. Additionally, routine CT imaging in donors and can add important data to inform the acceptance of organs for transplantation, such as excluding donor malignancies and better characterization of any abnormalities or pathology that may be present. To this end, work from our own institution has shown that CT imaging serves as a valuable adjunct in the evaluation of potential lung donors (16).
Recognizing the importance of accurate lung measurements for donors and recipients and the limitations of estimating lung volumes based on surrogate measurements, prior studies have developed predictive equations for lung volumes from CT analyses (11). These authors evaluated the CT scans of 400 normal patients and used 3D-CT volumetry to create mathematical models to predict right, left, and total lung volumes. They noted a strong correlation between CTvol and pTLC. In another study, Jung and colleagues (12) enrolled 264 patients in three groups (obstructive disease, restrictive disease, normal) and analyzed correlations between pTLC, plethysmography TLC, and CT vol and found that CTvol showed similar or better correlation with plethysmography TLC compared to pTLC in normal participants and patients with obstructive or restrictive disease. Similar to our findings, this study noted that CTvol was generally lower than pTLC. Fujimoto and colleagues (13) evaluated 53 LT recipients with CT volumetry and correlated CTvol measurements with donor pTLC. They noted that volumetric analysis in donors may help surgeons precisely downsize oversize lung grafts prior to LT. Our case series builds on prior work by prospectively utilizing CT volumetry in assessing suitability of a potential donor for a specific recipient.
The limitations of CT volumetry must be considered before effective translation into clinical transplantation is possible. First, there may be scenarios in which donor CT imaging is not available. However, CT imaging is ubiquitously obtained in the current practice of donor organ allocation and utilizing this already available imaging to measure lung volumes would circumvent the need for additional tests or imaging. Additionally, CT volumetry has gained increasing use in determining liver transplantation suitability and has been accepted as an accurate method for estimating liver volume (17,18). Therefore, this technology could be reasonably incorporated by radiologists for the measurement of lung volumes in addition to abdominal organ volumetry. Furthermore, factors such as mechanical ventilation settings may influence CT volumetric measurements. Ideally, CT volumetry is obtained with an inspiratory breath hold during scan acquisition. However, this may not always be possible in recipients with advanced lung disease and is not standard practice during donor CT scan acquisition at many organ procurement facilities. These variables may have slightly underestimated donor CTvol measurements in this study. Therefore, implementation of a CT scan acquisition protocol in organ donors to standardize mechanical ventilation settings as well as a breath-holding phase would help mitigate measurement error attributed to breathing motion and ensure accurate CTvol measurements. Previously reported methods have described successful CT image acquisition during continuous free-breathing with simultaneous collection of digital spirometry to generate accurate multi-dimensional CT reconstruction (19). Finally, our experience is limited to a small number of patients and additional studies are necessary to corroborate these findings.
Conclusions
This is an initial report of prospectively utilizing CT volumetry as an adjunct to decision-making regarding suitability of donor lungs. In these cases, CT volumetry facilitated the confident acceptance of donor lungs that were initially predicted to be oversized based on other clinical measures. Future investigation into the utilization of CT volumetry for effective donor and recipient size matching should focus on its impact on clinical outcomes after LT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1203/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1203/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1203/coif). DK served as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. RRH reports grants and personal fees from Bristol Myers Squibb, Mallinckrodt, UpToDate, CareDx, Natera, and Transmedics, outside of the submitted work. VP has served on panel discussions for PrecisCa, has a spouse who owns stock in Intuitive Surgical, and reports the following grants: I01 HX002475, R01HL146856, R01CA258681, and MATF. CCF is a board member and owns stock in OpComm, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent according to the Washington University School of Medicine institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eberlein M, Arnaoutakis GJ, Yarmus L, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant 2012;31:492-500. [Crossref] [PubMed]
- Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest 2012;141:451-60. [Crossref] [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233-40. [Crossref] [PubMed]
- Keeshan BC, Rossano JW, Beck N, et al. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pediatr Transplant 2015;19:294-300. [Crossref] [PubMed]
- Ouwens JP, van der Mark TW, van der Bij W, et al. Size matching in lung transplantation using predicted total lung capacity. Eur Respir J 2002;20:1419-22. [Crossref] [PubMed]
- Vazquez Guillamet R, Vazquez Guillamet MC, Rjob A, et al. Uncertainty analysis of chest X-ray lung height measurements and size matching for lung transplantation. J Thorac Dis 2022;14:1042-51. [Crossref] [PubMed]
- Riddell P, Ma J, Dunne B, et al. A simplified strategy for donor-recipient size-matching in lung transplant for interstitial lung disease. J Heart Lung Transplant 2021;40:1422-30. [Crossref] [PubMed]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993;6:5-40. [Crossref] [PubMed]
- Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J 1995;8:492-506. [Crossref] [PubMed]
- Chen F, Kubo T, Shoji T, et al. Comparison of pulmonary function test and computed tomography volumetry in living lung donors. J Heart Lung Transplant 2011;30:572-5. [Crossref] [PubMed]
- Konheim JA, Kon ZN, Pasrija C, et al. Predictive equations for lung volumes from computed tomography for size matching in pulmonary transplantation. J Thorac Cardiovasc Surg 2016;151:1163-9.e1. [Crossref] [PubMed]
- Jung WS, Haam S, Shin JM, et al. The feasibility of CT lung volume as a surrogate marker of donor-recipient size matching in lung transplantation. Medicine (Baltimore) 2016;95:e3957. [Crossref] [PubMed]
- Fujimoto R, Nakajima D, Tanaka S, et al. Efficacy of three-dimensional computed tomography volumetry for recipients in downsizing oversized grafts in brain-dead donor lung transplantation. Gen Thorac Cardiovasc Surg 2021;69:1112-7. [Crossref] [PubMed]
- Date H, Aoyama A, Hijiya K, et al. Outcomes of various transplant procedures (single, sparing, inverted) in living-donor lobar lung transplantation. J Thorac Cardiovasc Surg 2017;153:479-86. [Crossref] [PubMed]
- Chen F, Fujinaga T, Shoji T, et al. Perioperative assessment of oversized lobar graft downsizing in living-donor lobar lung transplantation using three-dimensional computed tomographic volumetry. Transpl Int 2010;23:e41-4. [Crossref] [PubMed]
- Gauthier JM, Bierhals AJ, Liu J, et al. Chest computed tomography imaging improves potential lung donor assessment. J Thorac Cardiovasc Surg 2019;157:1711-8.e1. [Crossref] [PubMed]
- Robb CL, Fowler KJ, Bierhals AJ, et al. Impact of Pretransplantation CT on Liver Donation in Potential Deceased Organ Donors. J Am Coll Surg 2022;234:166-75. [Crossref] [PubMed]
- Schiano TD, Bodian C, Schwartz ME, et al. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation 2000;69:545-50. [Crossref] [PubMed]
- Low DA, Nystrom M, Kalinin E, et al. A method for the reconstruction of four-dimensional synchronized CT scans acquired during free breathing. Med Phys 2003;30:1254-63. [Crossref] [PubMed]


