
A randomized controlled phase III trial protocol: perioperative pirfenidone therapy in patients with non-small cell lung cancer combined with idiopathic pulmonary fibrosis to confirm the preventative effect against postoperative acute exacerbation: the PIII-PEOPLE study (NEJ034)
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and lethal lung disease that is also a high-risk cohort of lung cancer development. Furthermore, acute exacerbation (AE) of pulmonary fibrosis, which is sometimes induced after any treatment for lung cancer, including surgery, chemotherapy, and radiotherapy, is also a fatal illness with no effective preventative measures reported. Lung cancer combined with IPF also shows high malignancy, with strong invasiveness generally (1). Thus, deciding on a treatment plan can be particularly challenging for patients with lung cancer combined with IPF.
Radical surgery is a proposed standard method of curing resectable non-small cell lung cancer (NSCLC). However, NSCLC patients with IPF show a poor prognosis after surgery. Indeed, the postoperative 5-year survival rate for stage I lung cancer with interstitial lung disease was reported to be 59% (2), compared with 82% for stage I lung cancer in UICC-7 (3). A large cohort retrospective study for surgical treatment for NSCLC combined with IPF revealed a 10.3% AE rate within 30 days post-surgery and a 43.9% mortality rate after AE (4). Unfortunately, no effective treatment in practice was validated for the prevention of AE until 2021, even after surgery (5) or in cases without lung cancer (6), except for perioperative pirfenidone therapy (PPT).
We previously validated the safety and the feasibility of PPT, which involves the administration of oral anti-fibrotic drugs before surgery and continues after surgery (7). PPT seemed effective for suppressing AE in a retrospective study (8), and its efficacy was validated in a multicenter single-arm phase II clinical trial [PEOPLE study, West Japan Oncology Group (WJOG) 6711L] (9). Furthermore, a recent retrospective study revealed that pirfenidone decreased the risk of lung cancer (hazard ratio 0.11) in patients with IPF (10), and lung cancer patients with PPT tended to show a better prognosis than those without PPT retrospectively (8), suggesting that PPT may improve the prognosis by decreasing the risk of perioperative AE and controlling IP progression and de novo lung cancer. In addition, in basic research, the supportive data on the anti-cancer effect of pirfenidone reported that pirfenidone leads to tumor suppression by immune cell infiltration (11). We present the following article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-535/rc).
Methods
Study design and objectives
We intend to conduct a phase III multicenter prospective randomized controlled clinical trial to validate the effect of PPT. This study was launched in December 2017, and enrollment of patients will close in September 2023 to recruit a statistically calculated 230 applicants. According to a large retrospective survey on IPF-associated lung cancer, a prediction score for the exacerbation rate was proposed based on the following seven risk factors (12): previous AE, 5 points; usual interstitial penumonia (UIP) pattern, 4 points; greater than or equal to segmentectomy, 4 points; prior steroid administration, 3 points; male sex, 3 points; Krebs von den Lungen-6 (KL-6) >1,000 U/mL, 2 points; and %vaital capacity (VC) <80%, 1 point. In this study, considering that only UIP/IPF complicated lung cancer was included, the majority of IPF patients are male, and patients with a history of AE or those with a history of steroid administration were excluded, the scores would be expected to be concentrated in the range of 7–14 points. The predicted exacerbation rate for a score of 7 is 3.3%, and for a score of 14 is 23.6%, yielding a median predicted exacerbation rate of 13.45%. In contrast, the exacerbation rate in patients who completed the protocol treatment in WJOG6711L was 2.8% (9). Based on the above, assuming that the exacerbation rate would be 13.45% in the control group and 2.8% in the treatment group, α =0.05 (both sides) and β =0.2, the number of patients required in each group is 110. Assuming that 5% of cases would drop out or have incomplete data, the target number of patients was set at 115 per group, for a total of 230 cases. This clinical trial was registered with the University Hospital Medical Information Network (UMIN) on October 15th, 2017 (UMIN000029411), and candidates were also recruited from the Japan Clinical Oncology Group (JCOG), West Japan Oncology Group (WJOG), and North East Japan Study Group (NEJSG), to which most of the Japanese central hospitals belonged. An initial version of the study protocol was approved on August 28th, 2017, and the latest version 1.3, was approved on June 4th, 2020. This was an investigator-initiated clinical trial and received no financial support from pharmaceutical companies. The Central Data Review Committee consisted of two thoracic surgeons (T.I. and S.Y.), two pulmonologists (K.K. and A.A.), and a radiologist (S.S.). In total, 77 institutions were included this trial; the details are listed in the Institutions section. The investigator at each site will obtain approval from their Institutional Review Board (IRB) before enrollment. After a major revision of the study protocol, it is necessary to re-consult the IRB of each institution and report to the NEJ034 trial office after receiving approval. Investigators at each site need to explain the concept of this trial to the patient and obtain their consent. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The investigator at each site will obtain approval from their Institutional Review Board (IRB) before enrollment. After a major revision of the study protocol, it is necessary to re-consult the IRB of each institution and report to the NEJ034 trial office after receiving approval. Investigators at each site need to explain the concept of this trial to the patient and obtain their consent.
Key eligibility criteria
The key eligibility criteria are as follows: age >20 years old, clinical stage 0-IIIA (UICC-8) resectable NSCLC or strongly suspected, IPF diagnosed by high-resolution computed tomography (CT), an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. All patients must sign informed consent forms approved by the review board of each institution. Diagnostic criteria of IPF are based on An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Briefly, all of the following (I) through (IV) (UIP pattern) or (I), (II), and (IV) (possible UIP pattern) must be satisfied in high resolution CT (13).
- Subpleural, lower lobe predominance
- Reticular shadow
- Honeycomb (with or without traction bronchiectasis)
- No findings that do not conform to the UIP pattern
The key exclusion criteria are as follows: a history of anatomical lung resection (registration is allowed if it has been more than 6 months since the surgical biopsy to diagnose IPF); a history of treatment for IPF within the 6 months before enrollment; a history of chemotherapy, radiotherapy, or both that included the lung in the radiation field; active infection; ongoing pregnancy; and other severe complications.
Treatment plan
The study schematic diagram is shown in Figure 1. Patients will be allocated to the PPT group or control group, the latter of which is allowed to apply any AE preventative treatment, excluding anti-fibrotic agents like pirfenidone. Surgery without any prevention is also allowed in the control group. The surgical method is part of the AE risk factor as a stratification factor. The protocol allowed to change of the surgical method to secure patient safety; although that must be informed in Electronic Data Capture (EDC), it is also included in the subanalysis as a background comparison of the risk score.
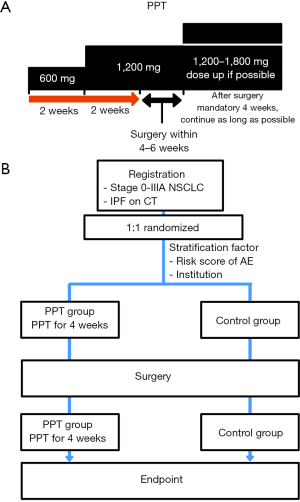
The stratification factors of this study are (I) institution and (II) the AE risk score that was previously verified (12). A subject will be assigned, using a computer program, to the PPT group or the control group by the NEJSG. In the PPT group, oral pirfenidone (600 mg) will be administered for 14 days after registration, and oral pirfenidone (1,200 mg) will be administered for over 14 days before surgery. Oral pirfenidone will then be resumed as soon as possible once the oral medication is allowed after surgery. The dose setting of pirfenidone was based on that of phase II clinical trial (WJOG6711L/PEOPLE study). According to the protocol, the drug administration should continue for at least 90 days after surgery in the PPT group, and after 90 days, be continued for as long as possible. Drug adherence will be monitored by investigators at each site and recorded. This trial is not a blind trial. In the control group, any AE preventative treatment excluding anti-fibrotic agents were allowed that depend on the policy of each institution (e.g., 1 week administration of sivelestat sodium hydrate in our institution). The control group also allows directly proceeding to surgery without any preoperative medication. Clinical information will be collected by EDC (Figure 2).
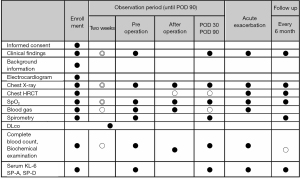
Endpoints
The primary endpoint is the IPF exacerbation rate within 30 days postoperatively. Secondary endpoints are as follows: (I) postoperative AE-free period (within 90 days); (II) safety information of complications due to pirfenidone and surgery; (III) the 2-year postoperative survival rate, recurrence-free survival rate of lung cancer, and progression-free survival rate of IPF; (IV) a background comparison using the risk score (12) for AE; (V) accuracy of the imaging diagnosis of IPF at the time of enrollment; and (VI) a subanalysis comparing the IPF and non-IPF cohorts decided by a central review of the CT findings. CT findings will be re-assessed by central review and decided on IPF or non-IPF. Before the analysis, all data will be collected by secured EDC (The Higgdb system provided by Zenbe, Japan), and data cleaning and periodical monitoring will be conducted by the NEJ034 trial office. Every 6 months, the clinical course and CT findings, including AEs, will be checked at the NEJ034 trial office to gather follow-up data until the patient reaches at least 2 years after the operation. All EDC data will be analyzed by the NEJ034 trial office. However, the statistical analysis will be conducted by an independent statistical manager.
The results of this study shall be the property of the NEJSG. The results obtained from this study will be reported regardless of the outcome. The Principal Investigator will select the authors of the paper according to the contribution of this trial.
Statistics
In this study, the following three target populations will be analyzed: the full analysis set (FAS), the per-protocol set (PPS), and the safety population (SP). The FAS will be the main analysis population, and all three types of target populations will be analyzed.
The primary endpoint, the rate of IPF exacerbations within 30 days after surgery, will be compared between groups. Fisher’s direct probability calculation method will be used for between-group comparisons. An exact logistic regression analysis will be performed to estimate odds ratios and 95% confidence intervals using the stratification factor as a covariate. For the various secondary endpoints, summary statistics will be calculated for each group, and between-group comparisons will be performed. A two-sample t-test will be used for between-group comparisons. In addition, an analysis of covariance will be used to compare the rate of change from baseline to each time point between groups. The covariate will be the baseline value. In addition, a linear mixed-effects model of longitudinal measurement will be applied as data analysis to show the change over time for each group, and compound symmetry (CS) will be used for the correlation structure. However, for binary data, the generalized estimating equation (GEE) method will be used. The cumulative number of events will be estimated by the Kaplan-Meier method, and survival curves will be generated for each group. Group comparisons will be examined using a stratified log-rank test, and hazard ratios and confidence intervals will be estimated using a Cox proportional hazards model with stratification factors as covariates to estimate effect sizes. The cumulative number of IPF AEs will be estimated by the cumulative incidence function method to generate a cumulative incidence curve for each group. Proof of the hypothesis will be examined with the Gray test. For estimation of the effect size, hazard ratios and their confidence intervals will be estimated using a competing risks regression model based on Fine-Gray’s proportional sub-hazards model with allocation adjustment factors as covariates. For the safety analysis, tabulations will be performed, and comments will be made for the safety endpoints. For interval estimation of proportions, exact two-sided 95% confidence intervals of the binomial distribution will be calculated for each group. If necessary, group comparisons will be made using Fisher’s direct probability calculation method. A randomized analysis will be considered for the protocol non-adherence population, and multiple imputations will be applied for handling missing data if needed, which will be conferred by the statistical analysis manager after fixing the statistical analysis plan.
Quality control
Central monitoring will be conducted based on the EDC/case report data collected at the NEJ034 trial office. If any problematic cases are found during the central monitoring, inquiries will be made to the relevant facility. For this periodic monitoring, the following items will be assessed: (I) compliance with case selection criteria (eligibility, etc.); (II) compliance with the protocol (deviation from the protocol, etc.); (III) responses to the occurrence of serious adverse events; (IV) status of occurrence of adverse events; (V) other safety issues; and (VI) omissions, inconsistencies, and other issues.
For safety reasons, the study will be terminated under the following situations. Once the decision to discontinue the study is made, the principal investigator will inform investigators at each site of the decision. (I) When the Efficacy and Safety Evaluation Committee recommends discontinuation of the study and the principal investigator determines that the study cannot be continued. (II) After the accumulation of 100 cases, the NEJ034 trial office will conduct safety monitoring to evaluate the safety and appropriateness of the study when data become available and submit a safety monitoring report to the Efficacy and Safety Evaluation Committee. The Efficacy and Safety Evaluation Committee will only evaluate the bias and safety of the enrolled procedures, review whether the study should continue, and—based on the results of the review—recommend changes to the protocol or discontinuation of the study. Based on the recommendation, the principal investigator and the NEJ034 trial office will decide to change the protocol or terminate the study. (III) If there is any other reason to terminate the entire study.
Adverse events are required to be reported within 15 days after they are recognized by investigators. Severe adverse events should be reported within 72 hours as a first report; then, the details will be described in the secondary statement within seven days after investigators recognize them. The Central Data Review Committee will be consulted in cases where an AE is suspected. If the Central Data Review Committee determines that an AE has occurred, it will be reported to the NEJ034 trial office, and once the number of AEs of IPF within 30 days exceeds 60, the Efficacy and Safety Committee will be consulted to determine whether the trial should continue from the perspective of patient protection.
The NEJSG office will conduct audits at the sites participating in the NEJSG study as necessary. In such cases, the designated person in charge of the audit will contact the participating sites in advance to arrange a visit for the audit. The responsible investigator at the study site and the head of the study site will ensure that the audit representative has access to all source documents related to the clinical research.
Discussion
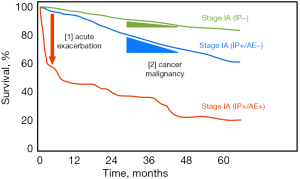
The aims of this study are shown in Figure 3. Both suppression of AE perioperatively and adequate surgical intervention to control lung cancer were required to achieve the ultimate goal of prolonging the patient survival. This study is designed to provide some evidence supporting the achievement of these goals. Perioperative AE suppression, which affects survival the most, is the main gist of this study. The primary endpoint is AE occurrence within 30-day, and additional information as AE occurrence within 90-day also observed as the secondary endpoint. In addition, this study will also follow patients for at least two years after surgery to evaluate the overall survival, cancer-free survival, and IP progression-free survival due to each perioperative management approach, including PPT. Recently, some new positive evidence of pirfenidone was reported for the chronic management of fibrosis (14-17), even though there is no perioperative evidence. We expect that these records will provide new, useful information to help manage NSCLC combined with IPF. The PIII-PEOPLE study will help validate the suppression effect of AE perioperatively and lead to the establishment of an optimized therapeutic strategy for NSCLC combined with IPF.
Acknowledgments
We thank all the PIII-PEOPLE study investigators who are participating in this study. This study is supported by Dr. Yasuo Saijyo (Niigata Univ.), Dr. Hiroyuki Shibata (Akita Univ.), and Dr. Koji Oba (Tokyo Univ.) as a data and safety monitoring committee, Mrs. Yuko Kikukawa, Mrs. Satomi Ishiwata, and other staff of the research organization NEJSG (North East Japan Study Group) as a clinical trial Clark.
Japan’s institutions were included in this clinical trial (Order from North to South). Hokkaido University Hospital, Asahikawa Medical University Hospital, TeineKeijinkai Hospital, Obihiro-Kosei General Hospital, Iwate Medical University Hospital, Tohoku University Hospital, Saka General Hospital, Akita University Hospital, Omagari Kousei Medical Center, Yamagata University Hospital, Fukushima Medical University Hospital, University of Tsukuba Hospital, Hitachi General Hospital, National Hospital Organization Ibarakihigashi National Hospital, Dokkyo Medical University Hospital, Jichi Medical University, Tochigi Cancer Center, Gunma University Hospital, Gunma Prefectural Cancer Center, Takasaki General Medical Center, Saitama Medical University International Medical Center, Saitama Cancer Center, Saitama Medical Center Jichi Medical University, Chiba Cancer Center, Chiba University Hospital, Nippon Medical School Chiba Hokusoh Hospital, National Cancer Center Hospital East, Tokyo Women’s Medical University Yachiyo Medical Center, The Jikei University Kashiwa Hospital, The Cancer Institute Hospital, Tokyo Medical University Hospital, The Jikei University Hospital, Toho University Omori Medical Center, Toranomon Hospital, Nippon Medical School Hospital, Tokyo Women’s Medical University Hospital, Tokyo Medical And Dental University Hospital, Kyorin University Hospital, The University of Tokyo Hospital, Showa University Hospital, National Hospital Organization Tokyo National Hospital, Japanese Red Cross Medical Center, Kanagawa Cancer Center, Kanagawa Cardiovascuiar and Respiratory Center, Showa University Northern Yokohama Hospital, Kitasato University Hospital, Sagamihara Kyodo Hospital, Niigata Cancer Center Hospital, Niigata University Graduate School of Medical and Dental Sciences, Kanazawa University Hospital, Kanazawa Medical University Hospital, Gifu University Hospital, Ogaki Municipal Hospital, Seirei Mikatahara General Hospital, International University of Health and Welfare Atami Hospital, Shimada General Medical Center, Kyoto University Hospital, Kindai University Hospital, Osaka City General Hospital, Osaka International Cancer Institute, Osaka University Hospital, National Hospital Organization Osaka Toneyama Medical Center, Kobe University Hospital, Kobe City Medical Center General Hospital, Hyogo Cancer Center, Tottori University Hospital, Okayama University Hospital, Hiroshima University Hospital, National Hospital Organization Kure Medical Center, Yamaguchi-Ube Medical Center, Tokushima University Hospital, National Hospital Organization Shikoku Cancer Center, Kyushu University Hospital, National Hospital Organization Kyushu Cancer Center, Nagasaki University Hospital, Kumamoto University Hospital, and Oita University Hospital.
Japan Medical Communication performed English proofreading.
Funding: This study is a self-funded trial under the support of the North East Japan Study Group (NEJSG).
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-535/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-535/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-535/coif). YS serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. AA has received research grant and personal fees from Boehringer Ingelheim, and grant from Taiho Pharmaceutical Co. Ltd., and personal fees from Toray and Kyorin Pharmaceutical Co. Ltd. KK has received personal fees from Takeda Pharmaceutical Co. Ltd. and AstraZeneca. IY has received grants, consulting and personal fees from AstraZeneca and Chugai, grant and personal fees from Taiho Pharmaceutical Co. Ltd., and personal fees from Johnson and Johnson, Covidien, Intuitive Surgical, MSD, and Shionogi. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hata A, Suzuki H, Nakajima T, et al. Concomitant Interstitial Lung Disease Is a Risk Factor for Pleural Invasion in Lung Cancer. Ann Thorac Surg 2017;103:967-74. [Crossref] [PubMed]
- Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64-9, 70.e1-2.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-11.e3. [Crossref] [PubMed]
- Iyoda A, Azuma Y, Sakamoto S, et al. Surgical treatment for patients with idiopathic pulmonary fibrosis and lung cancer: postoperative acute exacerbation of idiopathic pulmonary fibrosis and outcomes. Surg Today 2022;52:736-44. [Crossref] [PubMed]
- Meyer KC. Acute exacerbations of interstitial lung disease: what is the best treatment? Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021001. [PubMed]
- Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today 2015;45:1263-70. [Crossref] [PubMed]
- Iwata T, Yoshida S, Fujiwara T, et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2016;102:1905-10. [Crossref] [PubMed]
- Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [Crossref] [PubMed]
- Miura Y, Saito T, Tanaka T, et al. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Investig 2018;56:72-9. [Crossref] [PubMed]
- Marwitz S, Turkowski K, Nitschkowski D, et al. The Multi-Modal Effect of the Anti-fibrotic Drug Pirfenidone on NSCLC. Front Oncol 2019;9:1550. [Crossref] [PubMed]
- Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Krauss E, Tello S, Wilhelm J, et al. Assessing the Effectiveness of Pirfenidone in Idiopathic Pulmonary Fibrosis: Long-Term, Real-World Data from European IPF Registry (eurIPFreg). J Clin Med 2020;9:3763. [Crossref] [PubMed]
- Ley B, Swigris J, Day BM, et al. Pirfenidone Reduces Respiratory-related Hospitalizations in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2017;196:756-61. [Crossref] [PubMed]
- Finnerty JP, Ponnuswamy A, Dutta P, et al. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: a systematic review and meta-analysis. BMC Pulm Med 2021;21:411. [Crossref] [PubMed]
- Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, andomized, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147-57. [Crossref] [PubMed]


