
Simplified total arch reconstruction with a stented graft for extended aortic arch dilation
Highlight box
Key findings
• The s-TAR technique is a safe and effective alternative for total arch reconstruction with shorter operation time, lower rate of postoperative complications and lower total hospitalization costs compared with c-TAR.
What is known and what is new?
• Decades of experience have shown the conventional surgical procedure of aortic arch management to be complex, difficult and time-consuming.
• The new, simplified total arch reconstruction with a stented graft provides a safe alternative for extended aortic arch dilation treatment, with satisfactory operative results.
What is the implication, and what should change now?
• Our novel technique for total arch reconstruction has decreased circulatory arrest times and improved outcomes for patients.
Introduction
Aortal dilatation is defined as symmetrical enlargement of the aortic wall circumference (1). When the diameter exceeds the normal diameter by 50%, such dilatation is considered as an aneurysm (2). Patients presenting with thoracic aortic aneurysms are most commonly asymptomatic, and the aneurysmal aorta is usually detected by an astute primary care physician or cardiologist during routine chest X-ray, computed tomography (CT) scan, or echocardiography (3). An ascending aortic aneurysm with extended aortic arch dilation is a serious condition that requires surgical intervention to prevent aortic rupture or dissection (4). The most common treatment is simultaneous ascending aorta replacement and aortic arch reconstruction under hypothermic circulatory arrest (4).
The aortic arch and its three supra-aortic vessels remain a great surgical challenge for reconstruction (5). In China, Sun’s procedure is the conventional choice for total arch replacement (c-TAR), which has produced satisfactory early and long-term results since its introduction in 2003 (6). However, separate reimplantation of the three supra-aortic branches is a complex, traumatic technique, and the long cardiopulmonary bypass (CPB) and circulatory arrest times may also increase the risk of postoperative complications (7). Therefore, it is desirable to simplify the c-TAR procedure and still achieve good operative outcomes.
We designed a simplified total arch reconstruction with a modified stent graft (s-TAR), which eliminated the need for direct anastomoses of the arch graft with the three supra-aortic branches. The aim of this study was to compare its operative outcomes with those of the c-TAR procedure. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1283/rc).
Methods
Study design
This study was a retrospective analysis of prospectively collected data from all consecutive patients who had ascending aortic aneurysm with extended aortic arch dilation and underwent simultaneous ascending aorta replacement and aortic arch reconstruction with s-TAR or c-TAR between 2018 and 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of the First Affiliated Hospital of Army Medical University (Institutional Review Board File KY2021058). Written informed consent for intervention and data recording was signed by each patient.
The indication for intervention was a maximum diameter of the ascending aorta >55 mm and aortic arch in zone II >35 mm on CT angiography (CTA) (8). The exclusion criteria were: (I) primary tear involving the aortic arch or orifices of the three supra-aortic branches; (II) severe atherosclerosis in the aortic arch or severe calcified plaque in the origin of supra-aortic vessels that could increase the risk of endoleak and cerebral infarction; and (III) serious comorbidities such as ruptured aneurysm, severe coagulation disorder, and multiple organ failure.
All procedures were performed by two dedicated surgeons. The decision to proceed with s-TAR or c-TAR was discretionary based on the underlying clinical condition. In general, for each one patient from the s-TAR cohort, one control subject was recruited into the c-TAR cohort. The 1-to-1 matching was based on variables identified a priori to be of interest. Matching variables included age (±5 years), sex (exact), weight (±20 kg), height (±20 cm), and left ventricular ejection fraction (LVEF, ±10%).
Each patient underwent clinical examination, laboratory testing, and CTA at baseline, before discharge, 3 months after the surgery, and every 6 months thereafter. If renal function did not allow CTA, CT without contrast was performed. CTA imaging was assessed by a vascular radiological team. Data on baseline characteristics, comorbidities, concomitant cardiac surgeries, operative details, postoperative outcomes, and overall survival were collected prospectively and entered electronically into a dedicated Microsoft Access database.
Stent graft
The stent graft (Microport Medical Co., Ltd., Shanghai, China) consisted of polyester fabrics and a self-expandable metallic stent (diameter, 28–30 mm). A 10-mm stent-free sewing Dacron edge was placed on both ends, to which a conventional hand-sewn anastomosis could be done. We used a vascular prosthesis (Maquet Medical Equipment Co., Ltd., Shanghai, China) for ascending aorta replacement (diameter, 26–30 mm), and a 4-branched arch graft (Maquet Cardiovascular, Wayne, NJ, USA) for the c-TAR.
Surgical procedures
All procedures in this study were done under general anesthesia and via a standard median sternotomy (Figure 1). CPB was established by cannulating the femoral artery and placing a dual-stage atriocaval cannula in the right atrium. The distal ascending aorta was cross-clamped, and cold blood cardioplegia was infused intermittently in an antegrade fashion through the coronary ostia. During the cooling phase, the ascending aorta (and the aortic root or valve in some patients) was replaced and other concomitant procedures were done if indicated (Figure 1, A1,B1,A2,B2). After the proximal procedure was accomplished with achievement of the anticipated hypothermic circulatory arrest nasopharyngeal temperature of 27 °C, the circulation was arrested. Antegrade selective cerebral perfusion (SCP) was achieved through direct cannulation of the innominate artery at a flow rate of 8–10 mL/kg/min. When the left cerebral oxygen saturation was <50% tested by intraoperative near-infrared spectroscopy, the conversion of unilateral to bilateral SCP was initiated by the insertion of a separate cannula into the left common carotid artery.

S-TAR procedure
In the simplified procedure, the aortic arch was transected proximal to the origin of the innominate artery. The diameter of the native mid-arch (between the left carotid and left subclavian arteries) was measured by inserting a ball-shaped sizer to select the appropriate stent graft size. The stent graft was then inserted anterogradely into the aortic arch and descending aorta under direct visualization, and released once the proximal end of the self-expandable metallic stent was positioned just proximal to the inferior side of the orifice of the innominate artery. Subsequently, an occlusive balloon was inserted and deployed in the descending aorta to resume perfusion of the lower body via the femoral artery (Figure 1, C1,C2).
Three elliptical holes on the polyester fabric of the stent graft were separately modified around each arch branch orifice under direct visualization and using a pair of surgical scissors. The modification diameter at the stent graft was similar to that of each branch orifice (Figure 1, D1,D2). Next, each arch branch vessel was connected to the stent graft, using the inside-to-out technique, followed by the returning-to-the-inside technique, through all the layers of the polyester fabric and the native aortic arch wall as deeply as possible. Specifically, the polyester fabric of the stent graft at the base of the modification was first sutured around each arch branch orifice using a 5-0 polypropylene 17-mm 1/2C double-armed mattress suture. Next, the polyester fabric was stitched to the native aortic arch wall using a continuous suture. Usually, the left subclavian artery (LSA) was reconstructed first, followed by the left common carotid artery and then the innominate artery. After reconstructing the aortic arch, the proximal end of the stented graft and aortic wall were anastomosed to complete the proximal aortic arch stump plasty with a continuous suture (4-0 polypropylene suture) (Figure 1, E1,E2).
The distal end of the vessel prosthesis for replacement of the ascending aorta was trimmed to a suitable shape, and the end-to-end anastomosis between the proximal aortic arch containing the intraluminal stented graft and the distal ascending aortic prosthesis was accomplished with a continuous suture (4-0 polypropylene suture). To avoid tearing during anastomosis, a vascular graft strip was placed on the external wall to strengthen the aortic arch (Figure 1, F1,F2).
C-TAR procedure
The c-TAR refers to Sun’s procedure, which has been reported previously (6). Briefly, the three supra-aortic branches were extensively dissociated and the distal aorta was transected just proximal to the origin of the LSA. The stent graft, which was similar to that used in the s-TAR, was implanted in the descending aorta. The proximal end of the stented graft was positioned just distal to the origin of the LSA. The distal aorta containing the intraluminal stented graft was firmly anastomosed with the 4-branched arch graft, followed by resumption of lower-body perfusion through the perfusion limb of the 4-branched arch graft. Finally, the LSA, left common carotid artery, and innominate artery were anastomosed to the respective limbs of the 4-branched arch graft.
After rewarming, SCP was discontinued, and the systemic circulation was gradually restarted. The remainder of each procedure, including hemostasis and sternal closure, were performed routinely.
Outcomes
Primary outcomes were perioperative death and complications. Complications included prolonged ventilation, neurologic dysfunction, recurrent laryngeal nerve injury, chylothorax, paraplegia, acute renal failure, reoperation for bleeding, and endoleak requiring additional surgical treatment. Prolonged ventilation was the need for mechanical ventilation >48 h. Acute renal failure was defined as the need for dialysis. Neurologic dysfunction was diagnosed by neurologists based on their evaluations and relevant imaging (CT or diffusion magnetic resonance imaging). Transient neurologic dysfunction was defined as transient neurologic dysfunction including seizure and temporary cognitive or motor deficits that resolved completely within 3 months postoperatively. Conversely, permanent neurologic dysfunction was defined as stroke caused by cerebral infarction or hemorrhage that did not completely resolve.
Statistical analysis
Continuous variables are shown as the mean with standard deviation, and categorical variables are presented as percentages. Between-group differences were analyzed using Student’s t-test or the Mann-Whitney U-test for continuous variables and a chi-squared or Fisher’s exact test for categorical variables. Statistical analyses were performed using SPSS Statistics for Windows, Version 26.0 (IBM Corporation, Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 84 consecutive patients underwent simultaneous ascending aorta replacement and aortic arch reconstruction with the s-TAR (n=43) or c-TAR (n=41) between 2018 and 2021. The characteristics of both groups are reported in Table 1. No inter-group differences were found for sex, age, comorbidities, or EuroSCORE II results.
Table 1
| Variables | s-TAR (n=43) | c-TAR (n=41) | P value |
|---|---|---|---|
| Male: female | 28:15 | 27:14 | 0.889 |
| Age (years) | 52.5±12.4 | 53.0±10.7 | 0.885 |
| Body mass index (kg/m2) | 25.3±3.8 | 25.8±4.1 | 0.966 |
| Comorbidities | |||
| Hypertension | 6 (14.0) | 6 (14.6) | 0.893 |
| Diabetes mellitus | 4 (9.3) | 3 (7.3) | 0.658 |
| Dyslipidemia | 2 (4.7) | 2 (4.9) | 0.887 |
| Atrial fibrillation | 4 (9.3) | 4 (9.8) | 0.663 |
| Coronary artery disease | 2 (4.7) | 2 (4.9) | 0.884 |
| Chronic renal insufficiency | 3 (7.0) | 3 (7.3) | 0.775 |
| COPD | 6 (14.0) | 5 (12.2) | 0.557 |
| Stroke | 0 | 0 | 1.000 |
| Peripheral vascular disease | 0 | 0 | 1.000 |
| Marfan syndrome | 4 (9.3) | 3 (7.3) | 0.569 |
| Previous cardiac surgery | 1 (2.3) | 1 (2.4) | 0.545 |
| EuroSCORE II | 5.2±4.5 | 5.2±4.9 | 0.899 |
Values are expressed as mean ± standard deviation or number (%). s-TAR, simplified total arch reconstruction; c-TAR, conventional total arch replacement; COPD, chronic obstructive pulmonary disease.
Operative details
The operative details are presented in Table 2. All patients were effectively treated with the s-TAR or c-TAR procedure. No patient died intraoperatively, and no significant differences in the rate of concomitant procedures were found in both groups. Total operation, CPB, SCP, and lower-body circulatory arrest (LBCA) times were significantly shorter in the s-TAR group than in the c-TAR group.
Table 2
| Variables | s-TAR (n=43) | c-TAR (n=41) | P value |
|---|---|---|---|
| Concomitant procedures | |||
| Bentall procedure | 24 (55.8) | 23 (56.1) | 0.848 |
| Wheat’s procedure | 19 (44.2) | 18 (44.0) | 0.937 |
| Mitral valve replacement | 2 (4.7) | 2 (4.9) | 0.866 |
| Coronary artery bypass grafting | 1 (2.3) | 1 (2.4) | 0.625 |
| Procedural time | |||
| Total operative time (min) | 333.1±41.0 | 467.5±53.0 | 0.019 |
| CPB time (min) | 181.1±33.3 | 248.9±41.6 | 0.028 |
| Aortic cross-clamp time (min) | 114.6±24.0 | 157.5±31.0 | 0.026 |
| SCP time (min) | 32.3±3.7 | 44.2±5.2 | 0.045 |
| LBCA time (min) | 21.1±5.4 | 33.2±7.9 | 0.031 |
| Operative deaths | 0 | 0 | 1.000 |
Values are mean ± standard deviation or number (%). s-TAR, simplified total arch reconstruction; c-TAR, conventional total arch replacement; CPB, cardiopulmonary bypass; SCP, selective cerebral perfusion; LBCA, lower-body circulatory arrest.
Perioperative outcomes
The perioperative outcomes are summarized in Table 3. Prolonged ventilation was notably less in the s-TAR group. The incidence of recurrent laryngeal nerve injury and paraplegia was significantly increased in patients undergoing the c-TAR; however, no such events were observed in the s-TAR group. A new-onset chylothorax occurred in one patient of the c-TAR group, who recovered uneventfully with lipiodol lymphography and was discharged on postoperative day 16. The incidence of transient neurologic dysfunction was also significantly higher in the c-TAR group than in the s-TAR group, and all these patients fully recovered before discharge. No patient in either group experienced permanent neurologic dysfunction. The incidence of acute renal failure requiring dialysis showed a tendency to be higher in the c-TAR group, but the difference was not significant. Furthermore, perioperative blood loss and the incidence of reoperation for bleeding were significantly higher in the c-TAR group, which all resulted from surgical field errhysis due to a coagulation disorder rather than massive anastomotic hemorrhage. The in-hospital mortality rate was 0% in the s-TAR group and 4.9% in the c-TAR group, displaying a significant difference. In the c-TAR group, one patient died of low cardiac output syndrome after refusing further treatment on postoperative day 6, and the other patient died of cardiac arrest due to malignant arrhythmia on postoperative day 7. The s-TAR group had shorter ICU stay, shorter hospital stay and lower total hospitalization costs than the c-TAR group.
Table 3
| Variables | s-TAR (n=43) | c-TAR (n=39) | P value |
|---|---|---|---|
| Postoperative complications | |||
| Prolonged ventilation | 3 (7.0) | 7 (17.9) | 0.018 |
| Transient neurologic dysfunction | 2 (4.7) | 5 (12.8) | 0.025 |
| Permanent neurologic dysfunction | 0 | 0 | 1.000 |
| Recurrent laryngeal nerve injury | 0 | 3 (7.7) | 0.012 |
| Chylothorax | 0 | 1 (2.6) | 0.025 |
| Paraplegia | 0 | 2 (5.1) | 0.029 |
| Acute renal failure | 2 (4.7) | 2 (5.1) | 0.865 |
| Reoperation for bleeding | 1 (2.3) | 3 (7.7) | 0.934 |
| Endoleak | 0 | 0 | 1.000 |
| Perioperative blood loss (mL) | 1,112.6±680.9 | 1,889.9±807.7 | 0.024 |
| In-hospital death | 0 | 2 (4.9) | 0.035 |
| Length of ICU stay (days) | 2.7±1.0 | 5.4±2.0 | 0.031 |
| Length of hospital stay (days) | 9.7±2.9 | 14.7±5.9 | 0.037 |
| Total hospitalization cost (×105, yuan) | 14.5±3.2 | 19.5±4.8 | 0.035 |
Values are mean ± standard deviation or number (%). s-TAR, simplified total arch reconstruction; c-TAR, conventional total arch replacement; ICU, intensive care unit.
Overall survival
All patients were followed postoperatively up to December 2021 by telephone or direct interview. The average follow-up was similar in both groups (21.5±4.5 months for the s-TAR group and 20.5±5.5 months for the c-TAR group). The overall survival rate was 97.7% in the s-TAR group and 97.4% in the c-TAR group, and there were no aortic-related deaths. In the s-TAR group, one patient died of severe acute pancreatitis 10 months after discharge. In the c-TAR group, one patient with postoperative paraplegia died of multiple organ failure 8 months after discharge, and the other patient with postoperative paraplegia survived and recovered normal physical strength 19 months after discharge. Three patients in the c-TAR group who sustained recurrent laryngeal nerve injury remained hoarse. The two patients in each group with postoperative acute renal failure were still receiving regular dialysis. All other patients resumed their daily lives, and no patient had new neurologic dysfunction, paraplegia, or aortic-related reintervention at the time of analysis.
Imaging
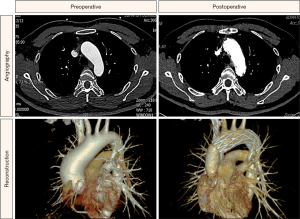
For all survivors, their aortic morphology was assessed by CTA during regular postoperative appointments. The maximal diameter changes at different aortic levels are shown in Table 4. The maximal diameter of the ascending aorta and aortic arch in zone II were significantly smaller at discharge than on admission, and there were few changes 3 months after discharge. No patient had modification site stenosis, endoleak, or dissection after the s-TAR procedure, and representative preoperative and postoperative aortic arch morphologies are shown in Figure 2.
Table 4
| Level of aorta | Group | On admission | At discharge | At 3 months |
|---|---|---|---|---|
| Ascending aorta (mm) | s-TAR | 54.3±3.5 | 27.7±2.5* | 26.9±2.5* |
| c-TAR | 54.0±3.6 | 28.1±2.3* | 27.5±2.7* | |
| Aortic arch in zone II (mm) | s-TAR | 38.8±2.9 | 29.3±2.4* | 28.5±2.1* |
| c-TAR | 38.5±3.1 | 29.5±2.6* | 29.0±2.5* |
Values are mean ± standard deviation. *P<0.05 vs. on admission. s-TAR, simplified total arch reconstruction; c-TAR, conventional total arch replacement.
Discussion
The standard contemporary surgical procedures for ascending aortic aneurysm with extended aortic arch dilation are the ascending aortic arch replacement combined with hemi- or total arch replacement and the classical elephant trunk or stented elephant trunk technique (9). The hemi-arch replacement is literally described as resection of the ascending aorta, and elimination of the aortic arch wall to the orifice of innominate artery in the greater curvature and the lesser curvature as much as possible (10). Given the remnant aortic pathologies after hemi-arch replacement, this procedure may potentially place the patient at excess risk of aortic dilation, dissection, rupture and thus reoperation (11). Therefore, we have not used this method since 2015. Total arch replacement involves separate anastomoses of the three supra-aortic branches and 4-branched arch graft and implantation of a stent graft in the descending aorta, namely Sun’s procedure, which we still use. However, it has several disadvantages (12). Firstly, it requires extensive dissociation of the three supra-aortic branches, which may cause thoracic duct and recurrent laryngeal nerve injury (13). Secondly, complex surgical skills are required. For instance, anastomosis of the descending aorta can be difficult because of the deep surgical field (14). Thirdly, the limbs of the 4-branched arch graft have a tendency to bend or undergo stenosis after closure owing to inappropriate trimming and positioning (15). Fourthly, the duration of total arch replacement remains very lengthy, particularly the SCP and LBCA times, which may lead to adverse outcomes of patient mortality and morbidity (16).
Nevertheless, Sun’s procedure is safe and acceptable under stable conditions. In our study, the outcomes of patients who underwent it (c-TAR) were encouraging and there was neither aortic-related death nor permanent neurologic dysfunction. However, it should not be performed in some patients according to our clinical experience. For instance, in patients with coagulation dysfunction, this procedure should not be used because of the increased number of anastomoses. Furthermore, in patients who need concomitant aortic root or valve surgery, this procedure should not be chosen as it may result in prolonged operation time. A shorter operative time is better, especially for patients with serious comorbidities.
In recent years, various surgical techniques have been formulated to simplify the c-TAR and proved to have encouraging outcomes (17). For instance, the triple-branched stent graft technique, which truly made the surgical procedure easier, time-saving and less invasive (18). However, that technique has some potential risks such as postoperative stent graft shifting or kinking, which may cause aortic occlusion or disruption. Hu et al. (19) promoted another technique to simplify the procedure of LSA reconstruction during total arch replacement, in which the LSA is reconstructed by removing a patch of the polyester fabric of the stent graft proximal to the origin of the LSA. However, the anastomoses of two additional supra-aortic branches are still difficult and time-consuming, which hampered extensive application of this technique. With our s-TAR technique, the anastomoses of the three supra-aortic branches are abolished, and the total operation, CPB, SCP, and LBCA times are significantly shorter than with the c-TAR. Firstly, our procedure is technically simpler than the c-TAR; secondly, the stent graft implantation, modification and suture are easily completed in 8–10 minutes; thirdly, the circulatory arrest time is dramatically decreased by eliminating the anastomoses of the three arch branches; and finally, the hemostasis time is also reduced because the single anastomosis between the proximal aortic arch containing the stented graft and the distal ascending aortic prosthesis is clearly exposed, and any bleeding point can be easily controlled.
Our technique significantly reduced the incidence of postoperative complications compared with the c-TAR. Foremost, our technique does not require resection or extensive dissociation of the three supra-aortic branches, avoiding thoracic duct and recurrent laryngeal nerve injury. Thus, no patient experienced new-onset laryngeal nerve injury or chylothorax in the s-TAR group. Furthermore, our technique does not require replacement of the three supra-aortic branches or distal end to-end anastomosis, because the total native aortic arch is preserved, avoiding the risk of bleeding at the anastomotic sites. Additionally, paraplegia is a serious complication and occurred in two patients in the c-TAR group. In addition, the prolonged duration of SCP and LBCA, extensive stent coverage of the intercostal arteries associated with a lower distal landing zone of the stent graft (Th 7–10) are strong risk factors of paraplegia (20). With our technique, the SCP and LBCA times are significantly shorter, and the proximal end of the stented graft is “moved forward” and positioned just proximal to the origin of the innominate artery, resulting in limited sacrifice of intercostal arteries and avoiding extensive coverage of the descending aorta. So, no cases of paraplegia occurred in the s-TAR group. Finally, the c-TAR procedure had higher total hospitalization costs than the s-TAR, not only for the cost of the 4-branched arch graft but also the prolonged ICU and hospital stays.
The size of the stent graft is an important factor for successful use of the s-TAR technique. The diameter of the selected stent graft should be 2 mm larger than that of the native mid-arch (between the left carotid and left subclavian arteries), which is measured during the operation (21). The diameter of the proximal descending aorta also needs to be taken into consideration. An oversized stent graft will stress the aortic wall and even lead to catastrophic rupture of the aorta. Furthermore, during the procedure of “modification” care should be taken to avoid injury to the orifices of the three supra-aortic branches, and the modified diameter should be similar to that of each branch orifice.
Endoleak from anastomotic bleeding remains an important complication of a stent graft technique (22). CTA should be scheduled for each patient before discharge, 3 months after surgery, and every 6 months thereafter to detect endoleak and malperfusion of the three supra-aortic vessels. For a small endoleak, no reintervention is required other than close follow-up. However, type I endoleak should be managed with early reintervention using embolization or a branched stent graft (23). In our early procedures, we did find that the margin of the LSA was usually the source of type I endoleak; thus, we updated our protocol of anastomosis in subsequent cases, which emphasized maximum penetration through all layers of the graft and the native aortic arch wall to confirm a tight attachment between the stent graft and native arch. Since then, type I endoleak has not occurred. In our study, all surviving patients undergoing the s-TAR were confirmed to be patent without any type of endoleak during follow-up.
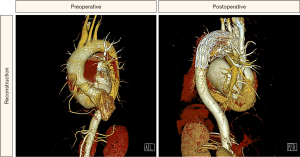
If the diameter discrepancy between the anastomosis site and zone II of the aortic arch is large, the s-TAR procedure can still be performed. When there is constant blood flow through the aortic arch, it creates pressure on the aortic wall and dilates the aortic arch. However, when the proximal end of the aortic aorta is blocked during the operation, the dilated aortic arch should shrink and thus the implanted stent graft can be tightly attached to the wall of the native aortic arch. Follow-up data from this study suggest that the stent graft was well attached to the native aortic arch. Based on our experience, selection of suitable patients is also key to the success of our s-TAR technique. If the distal aortic arch is >35 mm before operation, two stent grafts should be inserted, one of which is in the distal aortic arch and partially overlapped. In our clinical practice, there are a small number of patients who underwent the s-TAR procedure using two stent grafts due to the distal aortic arch being >35 mm, as shown in Figure 3.
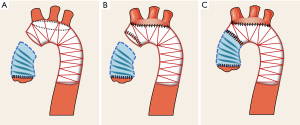
Actually, it is important for surgeons to measure the distance between the origins of three arch branch vessels on aortic CTA and it should be >1.5 cm for the s-TAR technique. During the procedure, the surgeon should reconfirm the distance before modifying the stent graft. If the distance between the origins of the three arch branch vessels is <1.5 cm, the s-TAR technique should adjust the “opening” protocol because of the high chance of endoleak. Specifically, the entire “island” opening on the polyester fabric of the stent graft is made around each of the orifices of the three arch branches under direct visualization and using a pair of surgical scissors. Thus, the three arch branch vessels are integrally sutured to the polyester fabric of the stent graft. The other steps are the same as before. Figure 4 is a schematic representation of the entire “island” modification in the s-TAR procedure.
Among healthy adults in China, the normal diameter of the aortic arch is ≈23.9–29.8 mm (24). A 50% increase is ≈35–45 mm, which is considered to be an arch aneurysmal dilatation. In the presence of an ascending aortic aneurysm, the diameter of aortic arch in zone II >35 mm could be considered an indication for concomitant aortic arch reconstruction. If the ascending aorta is replaced alone, the residual intact arch may be at excess risk of aortic dilation, dissection, and thus reoperation. In our early experience, patients with ascending aortic aneurysm and aortic arch dilation (zone II, 35–45 mm) commonly underwent ascending aorta replacement alone, not concomitant aortic arch reconstruction. Subsequently, a large proportion of the patients required secondary surgical management within 2–3 years because the residual aortic arch developed an aneurysmal dilatation or dissection (Figure S1). Therefore, prophylactic reconstruction of the intact aortic arch is necessary at the time of ascending aorta replacement for ascending aortic aneurysm combined with aortic arch dilatation as long as the patient’s condition permits this.
Study limitations
Patient allocation for each procedure was not done by randomization, so the surgeon’s preference or the patient’s anatomic suitability could be a potential bias of this study. In addition, this study was retrospective with a limited sample size. Further research with multiple centers and larger samples are scheduled.
Conclusions
The s-TAR technique is a safe and effective alternative for total aortic arch reconstruction with shorter operation time, lower rate of postoperative complications and lower total hospitalization costs compared with c-TAR.
Acknowledgments
Funding: This work was supported by the Foundation of Logistics Support Department (No. 20WQ004), and the Science and Technology Innovation Capacity Improvement Project of University (No. 2019XYY13).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1283/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1283/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1283/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1283/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the First Affiliated Hospital of Army Medical University (Institutional Review Board File KY2021058). Informed consent for intervention and data recording was signed by each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Obel LM, Diederichsen AC, Steffensen FH, et al. Population-Based Risk Factors for Ascending, Arch, Descending, and Abdominal Aortic Dilations for 60-74-Year-Old Individuals. J Am Coll Cardiol 2021;78:201-11. [Crossref] [PubMed]
- Ergin MA, Spielvogel D, Apaydin A, et al. Surgical treatment of the dilated ascending aorta: when and how? Ann Thorac Surg 1999;67:1834-9; discussion 1853-6. [Crossref] [PubMed]
- Ramlawi B, Little SH, Shah D. When to replace the ascending aorta? Methodist Debakey Cardiovasc J 2011;7:39-42. [Crossref] [PubMed]
- Spanos K, Nana P, von Kodolitsch Y, et al. Management of Ascending Aorta and Aortic Arch: Similarities and Differences Among Cardiovascular Guidelines. J Endovasc Ther 2022;29:667-77. [Crossref] [PubMed]
- Zhang B, Wei Y, Liu Y, et al. Safety and durability of single-stage type I hybrid total aortic arch repair for extensive aortic arch disease: early- and long-term clinical outcomes from a single center and our 10-year of experience. J Thorac Dis 2021;13:6230-9. [Crossref] [PubMed]
- Li Q, Ma WG, Sun LZ. Optimization of the total arch replacement technique: Left subclavian perfusion with sequential aortic reconstruction. J Thorac Cardiovasc Surg 2021;161:e447-51. [Crossref] [PubMed]
- Aoki H. Challenges in thoracic aortic aneurysm and dissection. J Thorac Dis 2018;10:S4140-3. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Murphy DL, Danielson KR, Knutson K, et al. Management of Acute Aortic Dissection During Critical Care Air Medical Transport. Air Med J 2020;39:291-5. [Crossref] [PubMed]
- Zhu C, Piao H, Wang Y, et al. A New Aortic Arch Inclusion Technique with Frozen Elephant Trunk for Aortic Arch Aneurysm Treatment. Int Heart J 2020;61:1229-35. [Crossref] [PubMed]
- Shelstad RC, Reeves JG, Yamanaka K, et al. Total Aortic Arch Replacement: Advantages of Varied Techniques. Semin Cardiothorac Vasc Anesth 2016;20:307-13. [Crossref] [PubMed]
- Wang H, Xu Z, Dai X, et al. Predicting postoperative hypoxemia risk factors in the patients after triple-branched stent graft implantation surgery with acute type A aortic dissection: A retrospective study. J Card Surg 2022;37:3642-50. [Crossref] [PubMed]
- Zhong L, Xiong H, Li J, et al. Early outcomes of Sun's procedure in elderly patients with acute aortic dissection: a single-center retrospective study. J Int Med Res 2022;50:3000605221109377. [Crossref] [PubMed]
- Yang S, Xue Y, Zhang YC, et al. Sun's total arch replacement and stent elephant trunk with modified branch-first technique for patients with Stanford type A aortic dissection. Ann Transl Med 2020;8:755. [Crossref] [PubMed]
- Huang Z, Zhang Z, Qiao G, et al. Application of the aortic balloon occlusion technique in Sun's procedure: A single-center study. J Card Surg 2022;37:1835-41. [Crossref] [PubMed]
- Bessho R. Neuroprotection during Open Aortic Arch Surgery: Cerebral Perfusion Methods and Temperature. J Nippon Med Sch 2023;90:11-9. [Crossref] [PubMed]
- D'Onofrio A, Caraffa R, Cibin G, et al. Total Endovascular Aortic Arch Repair: From Dream to Reality. Medicina (Kaunas) 2022;58:372. [Crossref] [PubMed]
- Morisaki A. Is open triple-branched stent graft the next stage? J Card Surg 2022;37:5218-9. [Crossref] [PubMed]
- Hu X, Wang Z, Ren Z, et al. Simplified total aortic arch replacement with an in situ stent graft fenestration technique for acute type A aortic dissection. J Vasc Surg 2017;66:711-7. [Crossref] [PubMed]
- Cuellar FL, Oberhuber A, Martens S, et al. Analysis of Spinal Ischemia after Frozen Elephant Trunk for Acute Aortic Dissection: An Observational, Single-Center Study. Diagnostics (Basel) 2022;12:2781. [Crossref] [PubMed]
- Pacini D, Murana G. The beauty of diversity: thoughts on different total arch repair. Eur J Cardiothorac Surg 2021;60:989-90. [Crossref] [PubMed]
- Liu Y, Zhang B, Liang S, et al. Early and Midterm Outcomes of Type II Hybrid Arch Repair for Complex Aortic Arch Pathology. Front Cardiovasc Med 2022;9:882783. [Crossref] [PubMed]
- Juan S, Liangtao X, Ligang L, et al. Application of Different Types of Hybrid Aortic Arch Repair: Toward to Solve Dissection Involving the Aortic Arch. Ann Vasc Surg 2022;83:222-30. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
(English Language Editor: K. Brown)


