
Efficacy and safety of immune checkpoint inhibitors versus chemotherapy in the second-line treatment of advanced esophageal squamous cell carcinoma: a meta-analysis and systematic review
Highlight box
Key findings
• Second-line therapy based on ICIs has better safety and efficacy than chemotherapy for patients with advanced ESCC.
What is known and what is new?
• In the past few years, the second-line treatment of advanced ESCC had gradually shifted from chemotherapy to ICIs, and the therapy of ICIs has had a certain effect.
• Our study demonstrated that ICIs have better safety and efficacy than chemotherapy in patients of advanced ESCC. A subgroup analysis of the study based on PD-L1 expression levels was also performed.
What is the implication, and what should change now?
• We conclude that ICI-based therapy is preferred for second-line treatment of patients with advanced ESCC, and the best treatment regimen needs to be selected according to PD-L1 expression levels.
Introduction
Esophageal cancer (EC) is a common digestive system disease, with a high incidence (604,000 new cases a year) worldwide. According to statistics, EC is prevalent in China, where the disease burden is the most significant (1). The 2 primary subtypes are esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC), and most cases of EC in Asia are ESCC (2). ESCC is mainly treated with surgical resection, radiotherapy, and chemotherapy. Traditional therapies have obvious limitations in the treatment process, and the results remain unsatisfactory (3). Advanced ESCC patients receive chemotherapy regimens based on taxane, platinum, and fluoropyrimidine as their first line of treatment as surgery is ineffective in this form of ESCC (4). However, for patients with advanced ESCC who show disease progression, there are few alternative second-line treatments. The primary immune checkpoint inhibitors (ICIs) that have demonstrated a considerable survival advantage over chemotherapy in numerous randomized controlled trials (RCTs) are programmed death ligand-1 (PD-L1) and programmed death 1 (PD-1) inhibitors, notably in advanced ESCC of which no response to first-line therapy is shown (5-9). In KEYNOTE-590, which investigated first-line therapy for advanced ESCC, pembrolizumab as one of the ICIs combined with chemotherapy improved overall survival (OS) in patients with advanced ESCC compared with placebo plus chemotherapy (10). A study discovered that ipilimumab, a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitor, in combination with nivolumab may be a possible therapy option, appropriate for patients with advanced ESCC (11), in addition to PD-L1/PD-1 inhibitors. This demonstrates how crucial ICIs are in the fight against cancer.
We aimed to explore the efficacy and safety of ICIs in the second-line treatment of advanced ESCC. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1169/rc) (12).
Methods
Search strategy and study selection
The PubMed, Embase, and Cochrane databases were searched for articles in English, on immunotherapy of ESCC reported from 1 January 2015 to 1 February 2022. The data searched also included unpublished information on immunotherapy for the patients of ESCC, from 1 January 2015 to 1 February 2022, at the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and other closely related conferences. The following keywords were used in the searching progress: “Esophageal Cancer”, “Esophagus Neoplasms”, “ESCC”, “ipilimumab”, “tislelizumab”, “sintilimab”, “nivolumab”, “pembrolizumab”, “camrelizumab”, “PD-1”, “CTLA-4”, “PD-L1”, and “randomized controlled trial”.
The medical trials that met the following inclusion criteria were included: (I) the study within the article showed that advanced ESCC is generally not suitable for surgical treatment; (II) ICIs for clinical trials had been approved by the Food and Drug Administration (FDA); (III) the report’s content was complete, primarily consisting of the research strategy, clinical information, and crucial data related to OS and progression-free survival (PFS), treatment-related adverse events (TRAEs), or objective response rate (ORR); (IV) the study group was divided into an immune group and a chemotherapy group based on different treatment methods; (V) English was the only language of publications; (VI) histologic type was ESCC. The exclusion criteria were as follows: (I) the disease was at an early stage; (II) there were no OS, PFS, and so on, among the important indicators of the study; (III) immunotherapy had been carried out before; (IV) the sample size was small, with no more than 10 cases; (V) the evaluation results related to safety and effectiveness were missing; (VI) repeated publication; (VII) other treatment methods were selected.
Endpoints
The primary endpoint of this study was the OS, which denoted the interval between the beginning of treatment and the mortality of any type. The secondary endpoints were PFS, ORR, and safety. PFS specifically referred to the period from the initiation of treatment to the disease’s progression or death. ORR measured how many patients achieve a complete or partial response (CR or PR, respectively). The term TRAE referred to any adverse reaction that occurred during treatment.
Quality assessment
The Cochrane manual was applied in the deviation risk study, and the quality of the study was evaluated based on the standards therein (13). In the process of quality evaluation, 2 experienced researchers conducted the evaluation independently. In case of inconsistent results, a decision was made based on the comprehensive evaluation conducted by a third party.
Statistical analysis
Meta-analysis was carried out based on the Review Manager 5.3 software tool (RevMan 5.3; The Nordic Cochrane Collaboration, Copenhagen Denmark). Before analyzing the results, the data of each outcome were extracted from each study. If there was only a 95% confidence interval (CI) and hazard ratio (HR), the inverse variance method was used for analysis. Based on the heterogeneity of the results, different models were selected to calculate these 2 indicators. The type of model was determined by the heterogeneity of the study. Heterogeneity was judged and analyzed by the chi-square test and quantified by a test of heterogeneity. Sensitivity analysis was performed to determine the cause of heterogeneity by removing studies with large deviations and then re-examining the heterogeneity. Publication bias was assessed according to Egger’s bias indicator test. Statistical data analysis was conducted using RevMan 5.3.
Results
Results of research
Following the literature search, we found 185 articles that matched our criteria. Some 60 articles were removed because of duplication and 105 were retrieved by reviewing the title and abstract. The remaining 20 articles were reviewed in full, and finally, 5 RCTs were selected for further analysis. The statistical results showed that 4 phase III and 1 randomized phase II study were included in the analysis. Of them, 5 studies were evaluated as high quality based on the assessment tool (Figure S1). Table 1 lists the features of the 5 studies included. The relevant procedure is displayed in Figure 1.
Table 1
| Study | Phase | Line of therapy | Arm | Patients | OS, HR (95% CI) | PFS, HR (95% CI) | Incidence of TRAEs | ORR | |
|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3–5 | ||||||||
| Kato 2019/ATTRACTION-3 (9) | III | 2 | Nivolumab 240 mg Q2W versus investigator choice chemotherapy | 210 versus 209 | 0.77 (0.62–0.96) | 1.08 (0.87–1.34) | 65% versus 94% | 18% versus 63% | 19% versus 22% |
| Huang 2020/ESCORT (8) | III | 2 | Camrelizumab 200 mg Q2W versus investigator choice chemotherapy | 228 versus 220 | 0.71 (0.57–0.88) | 0.69 (0.56–0.86) | 94% versus 90% | 19% versus 39% | 20.2% versus 6.4% |
| Kojima 2020/KEYNOTE-181 (7) | III | 2 | Pembrolizumab 200 mg Q3W versus investigator choice chemotherapy | 198 versus 203 | 0.78 (0.63–0.96) | 0.92 (0.75–1.13) | 64% versus 86% | 18.2% versus 40.9% | 16.7% versus 7.4% |
| Xu 2022/ORIENT-2 (6) | II | 2 | Sintilimab 200 mg Q3W versus investigator choice chemotherapy | 95 versus 95 | 0.70 (0.50–0.97) | 1.00 (0.72–1.39) | 54.3% versus 90.8% | 20.2% versus 39.1% | 12.6% versus 6.3% |
| Shen 2022/RATIONALE-302 (5) | III | 2 | Tislelizumab 200 mg Q3W versus investigator choice chemotherapy | 256 versus 256 | 0.70 (0.57–0.85) | 0.83 (0.67–1.01) | 73.3% versus 93.8% | 18.8% versus 58.8% | 20.3% versus 9.8% |
OS, overall survival; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; TRAEs, treatment-related adverse events; ORR, objective response rate; Q2W, 2 weeks using a; Q3W, 3 weeks using a.
Primary outcomes
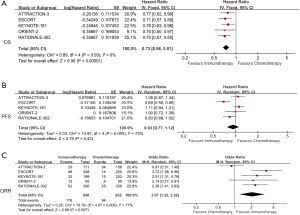
We extracted OS data from 5 studies for analysis (5-9). There was a significant reduction in death risk from ICIs in comparison to chemotherapy (HR =0.73; 95% CI: 0.66 to 0.81; P<0.001; I2=0%) (Figure 2A). We analyzed PFS data in these 5 studies; ICIs did not significantly differ from chemotherapy in terms of PFS, and the difference was not evident (HR =0.93; 95% CI: 0.77 to 1.12; P=0.43; I2=73%) (Figure 2B). ORR was also favorable in the ICIs group (OR =2.07; 95% CI: 1.22 to 3.52; P=0.007; I2=71%; P=0.008) (Figure 2C).
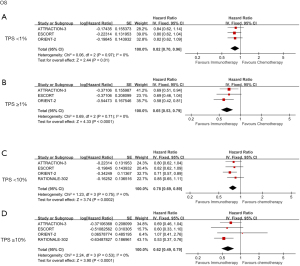
We further analyzed the relationship between PD-L1 expression and OS by classifying the combined positive score (CPS; sum of PD-L1 stained tumor cells and tumor-associated immune cells per 100 tumor cells) with tumor proportion score (TPS; percentage of PD-L1 membrane-stained tumor cells in tumor cells at any intensity) (14). ICIs showed better survival in patients with TPS <1% (HR =0.82; 95% CI: 0.70 to 0.96; P=0.01) and TPS ≥1% (HR =0.65; 95% CI: 0.53 to 0.79; P<0.001). However, the different expression levels created some differences between the 2 groups (Figure 3A,3B). Compared with the chemotherapy group, patients with TPS <10% and TPS ≥10% who received ICIs treatment showed OS benefits, with HRs of 0.78 (95% CI: 0.69 to 0.89; P=0.002) and 0.62 (95% CI: 0.49 to 0.79; P<0.001), respectively (Figure 3C,3D). Despite the differences in representation, patients with CPS <10 or CPS ≥10 had similar OS performance to TPS, and data from groups with higher CPS scores were more statistically significant in the analysis; their HRs were 0.9 and 0.66, P=0.38, and P=0.006, respectively (Figure 4A,4B). This data illustrates that the therapeutic effects of ICIs are different in different PD-L1 expression states.

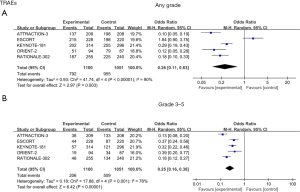
From the analysis of TRAEs, there were statistical differences observed in TRAEs of any grade [odds ratio (OR) =0.26; 95% CI: 0.11 to 0.63; P=0.003; I2=90%] (Figure 5A). However, after analyzing the data of grade 3–5 TRAEs, we found that the incidence of TRAEs in the ICIs group was significantly lower than that in the chemotherapy group (OR =0.25; 95% CI: 0.16 to 0.38; P<0.001; I2=78%), demonstrating that ICIs had a better safety profile (Figure 5B).
Sensitivity analysis and publication bias test
Sensitivity analysis was performed for each of the 5 studies, for HR of OS in the range of 0.72 to 0.74. We observed high heterogeneity in the results for PFS, ORR, and TRAEs and then performed sensitivity analyses individually. We identified the source of heterogeneity in PFS by including some studies and re-performed the statistical analysis, which showed no significant significance (HR =1.00; 95% CI: 0.87 to 1.16; P=0.95; I2=42%) (Figure S2). Repeat statistical analysis of ORR with the same method significantly reduced heterogeneity with statistical significance (OR =2.69; 95% CI: 1.95 to 3.70; P<0.001; I2=0%) (Figure S3). To Identify the heterogeneity of all TRAEs, we eliminated the studies with large deviations (OR =0.13; 95% CI: 0.09 to 0.20; P<0.001; I2=1%). Based on a re-sensitivity analysis of grades 3–5 TRAEs, ICIs still had a good safety profile in grade 3–5 (OR =0.35; 95% CI: 0.27 to 0.45; P<0.001; I2=0%) (Figure S4). Due to the small number of studies included in this analysis, publication bias could not be assessed.
Discussion
A growing number of studies have shown that immunotherapy can be effective in the treatment of EC. Immunotherapy involves inhibiting immune checkpoint pathways in the immune system to counteract malignant cells. A PD-L1 molecule binds to PD-1 receptors and induces PD-1 signaling, suppressing immune responses in T cells (15). The mechanism by which tumor cells evade immune response is mainly expression of the PD-1 ligand and activation of the PD-1 pathway based on the binding of corresponding effector cells to the PD-1 receptor. It can be inferred that the PD-1/PD-L1 pathway has important significance in immunotherapy and can be used as a therapeutic target in this regard. At present, the research tunnel related to this has received widespread attention. Previous studies had focused on ICIs, such as PD-1/PD-L1 inhibitors of ESCC (10,16). We evaluated the efficacy and safety of the currently published ICIs in the second-line treatment of advanced ESCC.
Our meta-analysis showed that ICIs significantly improved OS and ORR in the second-line treatment of advanced ESCC. However, the difference in PFS was not significant, which may be due to the longer duration of the immunotherapy (17). In our meta-analysis, there was a crossing of survival curves between ICI and chemotherapy, which means that the early benefit of chemotherapy is higher than that of ICI in some trials. The same results were observed in the KEYNOTE-061 study on gastroesophageal adenocarcinoma, which showed that the level of performance status was associated with survival benefit in subgroup analysis but lacked objectivity. It was further found that the survival curves for patients with a PD-L1 CPS of 10 or higher do not cross and the detrimental effect of immune checkpoint blockade on early progression appeared to be mitigated (18). In KEYNOTE-181, we found a significant benefit for both PFS and OS in patients with CPS of 10 or higher and no crossing of survival curves. Because the overall population is counted in many studies and subgroup analyses are not well performed, there may be situations where there is no PFS benefit but a survival benefit. The proportion of grades 3–5 TRAEs seems to be much lower in the ICI group. However, the incidence of the 2 groups had no significant difference. A significant finding of our study was that high levels of PD-L1 expression were associated with improved OS. The efficacy of the relative inhibitors was also associated with high PD-L1 levels. However, analysis of PD-L1 expression is not standardized, and inflammation can induce PD-L1 in tumors previously negative for such kind of marker (19). In one study, pembrolizumab was associated with a median OS of 9.3 months whereas chemotherapy resulted in a median OS of 6.7 months (7). Therefore, pembrolizumab was a better second-line standard therapeutic option for ESCC with high-level PD-L1. The ESCORT-1st trial results also indicated that patients with TPS ≥1% benefitted more from the treatment (20). The prediction of PD-L1 expression and prognosis warrants further study.
ICIs are generally better tolerated than chemotherapy but still have their own adverse effects (14). Grade 3–5 adverse events occurred in 18% of patients receiving pembrolizumab (7). Hypothyroidism (11.5%) and decreased appetite (8.6%) were the most common side effects. Diarrhea (10%), decreased appetite (7%), and fatigue (7%) were the most common TRAEs in the nivolumab group (9). The highest incidence of TRAEs caused by camrelizumab was 89%, and the most prominent TRAE was reactive cutaneous capillary endothelial proliferation in 79% of cases (8). In many groups, hypothyroidism was prevalent, but in the nivolumab group, it was less prevalent (5-9). According to our research, the incidence of grade 3–5 TRAEs following treatment with ICIs was lower than it was for the chemotherapy group, thus indicating that ICIs have a better safety profile than chemotherapy. Clinical trials on advanced ESCC are continuing. In the Checkmate 648 trial (11), the combination of PD-1 and CTLA-4 for advanced ESCC showed good efficacy. Additionally, 32% of patients experienced adverse responses of grade 3 or higher, and median response times of the patients with PD-L1-positive lasted much longer than those of chemotherapy. This shows that dual ICIs, especially in combination, may offer better benefits in treating advanced ESCC. The effect of PD-L1 expression on treatment outcomes has also been further investigated in the checkmate648 trial (11). In patients with TPS ≥1% or higher, both immune combination regimens had a better survival benefit compared with chemotherapy. In patients with TPS ≤1%, no significant survival difference was observed between the regimens, but the proportion of patients with objective response to immune combination therapy was more than that in the chemotherapy group, indicating that longer follow-up may be required to determine changes in OS. Similar results were also obtained in comparing patients with CPS ≥1 and CPS <1. These findings showed that both TPS and CPS have certain guiding significance for advanced ESCC. However, in gastroesophageal adenocarcinoma, CPS was found to be a more appropriate indicator than TPS (21). A TPS of 5% had a longer duration of response compared with 10%, and there was no significant difference in OS, suggesting that the benefit in a population with tumor cell TPS ≥1% may not be driven by a subgroup with TPS ≥10% (22,23). In a study of patients with advanced squamous non-small cell lung cancer (24), TPS ≥50% was associated with a greater OS benefit with nivolumab in the second-line treatment, and different immunotherapy regimens may be explored in the future based on different PD-L1 expression levels.
The limitations of the study are that it included only 5 studies with limited numbers of patients. The analyses of TPS and CPS, the 2 indicators of PD-L1 expression, were not standardized in each of the studies. Therefore, we cannot correctly assess the consistency of their predictive values.
Conclusions
According to the results of this study, ICIs can significantly improve the survival of patients with advanced ESCC while maintaining high safety standards, and the relevant results can guide the clinical treatment of this disease.
Acknowledgments
The authors thank Taizhou Hospital for the support of the study environment.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1169/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1169/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1169/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Rogers JE, Sewastjanow-Silva M, Waters RE, et al. Esophageal cancer: emerging therapeutics. Expert Opin Ther Targets 2022;26:107-17. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Muro K, Lordick F, Tsushima T, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:34-43. [Crossref] [PubMed]
- Shen L, Kato K, Kim SB, et al. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J Clin Oncol 2022;40:3065-76. [Crossref] [PubMed]
- Xu J, Li Y, Fan Q, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2). Nat Commun 2022;13:857. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Thuss-Patience P, Stein A. Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr Oncol 2022;29:2461-71. [Crossref] [PubMed]
- Theivanthiran B, Evans KS, DeVito NC, et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest 2020;130:2570-86. [Crossref] [PubMed]
- Fujiwara Y, Iguchi H, Yamamoto N, et al. Tolerability and efficacy of durvalumab in Japanese patients with advanced solid tumors. Cancer Sci 2019;110:1715-23. [Crossref] [PubMed]
- Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019;30:970-6. [Crossref] [PubMed]
- Smyth EC, Petty RD. Pembrolizumab versus paclitaxel in gastro-oesophageal adenocarcinoma. Lancet 2018;392:97-8. [Crossref] [PubMed]
- Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 2021;18:345-62. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Lei M, Siemers NO, Pandya D, et al. Analyses of PD-L1 and Inflammatory Gene Expression Association with Efficacy of Nivolumab ± Ipilimumab in Gastric Cancer/Gastroesophageal Junction Cancer. Clin Cancer Res 2021;27:3926-35. [Crossref] [PubMed]
- Kato K, Doki Y, Ura T, et al. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci 2020;111:1676-84. [Crossref] [PubMed]
- Lee J, Kim B, Jung HA, et al. Nivolumab for esophageal squamous cell carcinoma and the predictive role of PD-L1 or CD8 expression in its therapeutic effect. Cancer Immunol Immunother 2021;70:1203-11. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
(English Language Editor: J. Jones)


