
Improved complete portal 4-port robotic lobectomy for lung cancer: Hamamatsu Method KAI
Introduction
Robot-assisted thoracoscopic surgery (RATS) has been adopted rapidly in the last decade worldwide. Similarly, in Japan, the number of patients undergoing RATS lobectomy is increasing, with RATS lobectomy rapidly replacing traditional video-assisted thoracoscopic surgery (VATS). This is because RATS using the da Vinci Surgical System® (Intuitive Surgical Inc., Sunnyvale, CA, USA) has been covered by the medical insurance system since April 2018. Prior to this time, 76% of lung cancer resections in Japan were performed via VATS (1).
There are two main VATS techniques in Japan. One is “Himeji-style VATS” with a look-up view, developed by Miyamoto. In this technique the camera is inserted into the chest cavity from the caudal intercostal space (2). The other VATS technique is the “confronting upside-down monitor setting type” developed by Kohno (3) and Mun (4), wherein the camera is inserted from the upper intercostal space. While both technique are good VATS methods, the “confronting upside-down monitor setting type” is better for the cranial field of view. Furthermore, since the “confronting upside-down monitor setting type” shows the exact same field of view as in open thoracotomy, the transition from open thoracotomy to VATS is smooth. Sakakura et al. (5) also proposed a RATS approach with views similar to those in open thoracotomy. Thoracic surgeons performing VATS with the “confronting upside-down monitor setting type” have expressed interest in performing RATS with a similar perspective, such as that in Yamazaki et al.’s (6) report of an original anterior approach. The RATS method of Cerfolio et al. (7) uses a look-up view in which the camera enters from the lower intercostal space, similar to “Himeji-style VATS” (2). RATS techniques that utilize a more caudal camera port provide inferior visualization of the apex of the chest.
Thus, we previously established a new port arrangement, the “Hamamatsu Method”, to provide good cranial field of view using the da Vinci Xi surgical system (8). However, our VATS technique requires four ports, while the “Hamamatsu Method” requires five ports, including the assist port. Thus, we developed a new method called the “Hamamatsu Method KAI”, while maintains full functionality of all four robotic arms and the assistant. ‘KAI’ is a suffix in Japanese meaning ‘sequel’ or ‘successor’.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hamamatsu University School of Medicine (No. 16-185, 22-058). All study participants provided informed consent.
We analyzed all robotic lung cancer surgeries performed in our department from October 2017 to May 2022. We compared the safety aspects of “Hamamatsu Method KAI” with those of conventional 5- or 6-port robotic surgery. Conventional 5- or 6-port robotic surgery involves 4 robot ports and 1 or 2 assist ports. IBM SPSS was used for statistical analysis, and Mann-Whitney U test was used for between-group comparisons.
Surgical technique
Port placement (right upper lobectomy) (Figure 1)
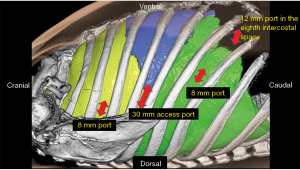
- First, a 30 mm access port was placed on the posterior axillary line in the 5th intercostal space. An alnote® lap single (Alfresa Pharma Corporation, Osaka, Japan) was attached to the access port (Figure 2), and an AirSeal® (Conmed Japan K.K., Tokyo, Japan) was passed into the lap single. Carbon dioxide (CO2) was pumped at 8 mmHg. An 8 mm port was also passed into the lap single for robotic arm 2. This was used for the camera. If bleeding occurred, this 30-mm access port was enlarged for emergency thoracotomy. The resected lung could also be removed through this 30-mm access port.
- The second port was a 12-mm port on the anterior axillary line in the 8th intercostal space. This port was inserted into the thoracic cavity while observing the insertion with a camera under artificial pneumothorax using CO2 insufflation. This port was docked to robotic arm 4 and was used for the robotic stapler and to provide retraction. This is the second right hand port.
- At 8 cm caudal to the access port also in the 7th or 8th intercostal space, an 8 mm port was placed for robotic arm 3. This port was handled by the surgeon’s right hand.
- The fourth port was an 8 mm port on the ventral side of the scapula in the 3rd dorsal intercostal space. This port was handled by the surgeon’s left hand.
Key considerations
- Using boom rotation of the da Vinci Xi surgical system, the patient’s cart was always inserted straight from the head side, irrespective of whether the patient was in the left or right lateral decubitus or supine position.
- Since the port of the anterior axillary line is close to the diaphragm attachment, it should be inserted while observing the insertion from inside the thoracic cavity.
- The patient’s right arm should be positioned, such that the shoulder blades are as far back as possible.
- An 8-mm robotic port should be placed on the ventral edge of the 30-mm access port and supported by an assistant from the dorsal side.
Comments
Among 20 patients with lung cancer who underwent anatomical lung resection with mediastinal lymph node dissection using the “Hamamatsu Method KAI”, there was no significant difference in bleeding volume (7.5 vs. 15 mL), drain placement period (2 vs. 3 days), operation time (206 vs. 210 min), and console time (137 vs. 162 min) compared to patients who underwent conventional 5-port surgery (n=57). No case of conversion to thoracotomy was detected among patients in the Hamamatsu Method KAI group (0 vs. 6). Additional information is shown in Table 1.
Table 1
| Parameters | Hamamatsu Methods KAI (n=20) | Robotic surgery with 5 or 6 ports (n=57) | P value |
|---|---|---|---|
| Age (years) | 68 [42–81] | 70 [47–83] | 0.253 |
| Total operation time (min) | 206 [146–369] | 210 [148–403] | 0.685 |
| Console time (min) | 137 [97–290] | 162 [99–277] | 0.174 |
| Bleeding volume (mL) | 7.5 [0–40] | 15 [0–175] | 0.090 |
| Conversion to thoracotomy, n | 0 | 6 | 0.240 |
| Post-operative hospital stay (days) | 7 [3–9] | 8 [4–17] | 0.197 |
| Drain placement period (days) | 2 [2–7] | 3 [1–13] | 0.787 |
| Complications of IIIB, n (%) | 4 (20.0) | 13 (22.8) | 0.893 |
All values are expressed as medians [range] or n (%).
In a standard surgery for lung cancer, either VATS or RATS, surgical specimens must eventually be removed from the chest cavity, which necessitates an incision of a few centimeters. Ninan and Dylewski (9) were the first people to popularize robotic lobectomy using three robotic arms, without intercostal access thoracotomy. In this form of lobectomy, the surgical specimen is removed through the transdiaphragmatic subcostal access. However, if a small incision is required to remove a surgical specimen, it is more convenient to use it as a utility incision. Later, Cerfolio et al. (7) described completely portal robot lobectomy with four arms (CPRL-4), which use all robotic arms and an assistant port. This technique provided a slightly modified, complete portal robotic approach using three arms developed and championed by Ninan and Dylewski (9) and requires five ports, including an assistant access port. Our previously reported “Hamamatsu Method” also uses four robotic ports and one assist port, making it a 5-port operation. However, we usually perform thoracoscopic lobectomy using four ports. We believe the number of ports in robotic lobectomy should not exceed those in VATS to preserve the advantage of minimal invasiveness. Furthermore, patients are generally more sensitive to wound size and number than surgeons assume. Therefore, we devised a method to reduce the number of ports without degrading the quality of the robotic surgery. CO2 insufflation is essential in both “Hamamatsu Method” and “Hamamatsu Method KAI” to secure a working space for forceps and cameras in the thoracic cavity. The AirSeal® system and the alnote® lap single can help maintain CO2 effectively while maintaining assistant access.
The reason for integrating the access and camera ports in Hamamatsu Method KAI is that the camera port requires/involves the least movement, compared to the other ports. Moreover, we reduced the risk of the robot arm hitting the assisting table surgeon by connecting the camera port to the access port. The camera is inserted from the ventral edge of the access port to avoid challenges in handling. In addition, although the access and camera ports are combined, the mobility of the three robot arms is completely unaffected.
The “Hamamatsu Method KAI” has certain limitations. In our study, the two patients groups were not randomized. Moreover, the “Hamamatsu Method KAI” is a recently developed surgical technique. Based on our results, we cannot definitively state that the “Hamamatsu Method KAI” is superior to existing methods. In contrast, we believe that the “Hamamatsu Method KAI” is not significantly inferior to the conventional method, and a validation study is required to compare it with the conventional method. Moreover, pain associated with surgery should be considered.
“Hamamatsu Method KAI”, an improved version of the conventional “Hamamatsu Method”, is an epoch-making RATS technique that enables 4-port surgery by reducing the number of ports while maintaining full functionality of all four robotic arms and the assistant. The novelty of this operative method is the combined camera/assistant/access incision, and this technique is an option for RATS for lung cancer.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing.
Funding: None.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1103/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1103/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hamamatsu University School of Medicine (No. 16-185, 22-058). All study participants provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2018: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2021;69:179-212. [Crossref] [PubMed]
- Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg 2010;89:353-9. [Crossref] [PubMed]
- Kohno T, Mun M. The advantage of video-assisted thoracic surgery in general thoracic surgery. Nihon Geka Gakkai Zasshi 2005;106:307-12. [PubMed]
- Mun M, Ichinose J, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg 2017;3:129. [Crossref] [PubMed]
- Sakakura N, Nakada T, Shirai S, et al. Robotic open-thoracotomy-view approach using vertical port placement and confronting monitor setting. Interact Cardiovasc Thorac Surg 2021;33:60-7. [Crossref] [PubMed]
- Yamazaki K, Toyokawa G, Shoji F, et al. A novel technique for robotic-assisted lobectomy for lung cancer: the anterior approach. Interact Cardiovasc Thorac Surg 2020;30:328. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7. [Crossref] [PubMed]
- Funai K, Kawase A, Mizuno K, et al. Uniquely Modified Robotic-Assisted Thoracic Surgery With Good Intrathoracic Visual Field. Ann Thorac Surg 2020;110:e435-6. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]



