Evidence and controversies regarding the screening for subclinical hypothyroidism in patients with cardiovascular disease
Subclinical hypothyroidism is defined as thyroid-stimulating hormone (TSH) level above the upper limit of the reference ranges with normal free thyroxine (T4) concentrations (1). Current data suggest that the prevalence of subclinical hypothyroidism can reach up to 10% in the elderly (2) and around 30% of subject can progress to overt hypothyroidism (low levels of T4) depending on the initial serum TSH concentrations and the presence of anti-thyroid peroxidase (anti-TPO) antibodies (3). Thyroid hormones have different effects on the cardiovascular system (4). Subclinical hypothyroidism is a well-known secondary reversible cause of hypercholesterolemia. Unspecific cardiac alterations have been associated with overt and subclinical hypothyroidism, such as impaired systolic function and left ventricular diastolic filling, increased peripheral vascular resistance, diastolic hypertension, increased arterial stiffness, endothelial dysfunction, pericardial effusion and arrhythmia (5-8). These findings are more likely to be observed in patients with higher TSH levels and in elderly.
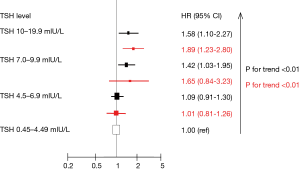
Data from observational prospective studies suggest an association between subclinical hypothyroidism and cardiovascular disease (CVD) events, while most of the available evidence derived from the consortium called “The Thyroid Studies Collaboration” (Figure 1) (9-11). After a systematic review, all eligible prospective cohorts were identified and their authors were contacted to share their data for an individual participant data (IPD) analysis. This process has the major strength to avoid aggregation-bias, as it might occur by study-level meta-analysis, as well as the following advantages: (I) increased statistical power; (II) adjustment with standardized confounding factors across studies; (III) definition of uniform TSH ranges; and (IV) use of similar clinical outcomes definition (12). Data from 11 prospective cohorts, including 55,287 patients and 3,450 (6.2%) with subclinical hypothyroidism showed higher rates of total coronary heart disease (CHD) mortality in those with higher TSH levels, especially for TSH levels of 7.0 to 9.9 mIU/L with hazard ratios (HRs) of 1.42 [95% confidence intervals (CI), 1.03–1.95] and for TSH levels ≥10.0 mIU/L with HRs of 1.58 (95% CI, 1.10–2.27, P for trend =0.005, Figure 2) (9). The HRs for CHD events were 1.17 (95% CI, 0.96–1.43) for TSH levels of 7.0 to 9.9 mIU/L and for TSH levels of 10 to 19.9 mIU/L (HR =1.89; 95% CI, 1.28–2.80; P<0.001 for trend) (10). No significant interaction was found according the presence of CVD at baseline. Using the same data in the assessment of heart failure (HF) events, the IPD of 6 prospective cohorts including 25,390 subjects (22,674 with euthyroidism and 2,068 with subclinical hypothyroidism) showed also higher risk of HF events: HR was 1.65 (95% CI, 0.84–3.23) for TSH levels of 7.0 to 9.9 mIU/L and 1.89 (95% CI, 1.23–2.80) for TSH 10–19.9 mIU/L (P for trend <0.01). No interaction was found also according to the presence of CVD at baseline.
These findings derived mainly from community-dwelling cohorts and few data were available in high-risk patients, such as those who were treated with percutaneous coronary intervention (PCI) (13). Revascularization with PCI remains the recommended therapies of acute coronary syndromes (ACS) or in patients with stable CAD presenting angina and with documented large ischaemia or significant coronary disease (14,15). Secondary prevention is especially important in this setting as the identification of emergent CVD risk, such as subclinical hypothyroidism, was poorly studied. In their article, Zhang et al., from the Mayo Clinic, reported among 2,430 patients treated with PCI an association between subclinical hypothyroidism and the occurrence of major adverse cardiovascular and cerebral events (MACCE) (13). Adjusted HRs were 1.28 (95% CI, 1.13–1.45; P=0.0001) for MACCE, HR 1.14 (95% CI, 0.75–1.69; P=0.54) for cardiac death, HR =1.25 (95% CI, 1.01–1.53; P=0.037) for myocardial infarction, HR =1.46 (95% CI, 1.13–1.88; P=0.004) for HF, HR =1.26 (95% CI, 1.10–1.43; P=0.0008) for revascularization and HR =1.62 (95% CI, 1.04–2.49; P=0.04) for stroke. Prevalence of subclinical hypothyroidism was quite high (28.2%) compared to data from the Thyroid Studies Collaboration, but the magnitude of the risk estimates was similar. In fact, as most patients had missing data for free thyroxine levels, distinguishing overt (low T4 levels) from subclinical hypothyroidism (normal T4 levels) was not feasible. Patients who had an appropriate thyroid replacement therapy with normalization of TSH levels had lower risk of MACCE compared to those with inadequate thyroid replacement (HR =0.78; 95% CI, 0.61-0.99; P=0.045) or those who remained untreated (HR =0.69; 95% CI, 0.52-0.89; P=0.005). Lower risks in patients adequately treated were also observed for cardiac death (P=0.008), myocardial infarction (P=0.004), HF (P=0.02), revascularization (P=0.41) and stroke events (P=0.01). Similarly, Ravzi et al. reported an association between thyroid replacement therapy and lower incidence of CHD events (adjusted HR =0.61; 95% CI, 0.39–0.95) among 3,093 patients with subclinical hypothyroidism (16). Although, no causality can be assumed due to the non-randomized design, these data suggest that treatment of subclinical hypothyroidism might be safe and possibly reduce the recurrence of major adverse cardiovascular events.
Currently the evidence to make a recommendation for thyroid replacement is not strong (level B). No adequately powered randomized controlled trial has investigated the impact of thyroid replacement therapy on cardiovascular outcomes. European Society of Cardiology (ESC) and American Heart Association (AHA) guidelines for the management of ACS did not address any specific issues regarding the screening and treatment of subclinical hypothyroidism (15,17). In 2012, AHA guidelines for the management of HF recommended to measure TSH levels in the assessment or progression of HF, but did not mention a specific threshold for thyroid replacement (18). Recently, the 2015 US Preventive Services Task Force for screening for thyroid dysfunction concluded that the current evidence was insufficient to assess the balance of benefits and harms of screening and treatment for thyroid dysfunction in asymptomatic adults, mainly due to the absence of large randomized controlled studies with clinical endpoints (19). Given the recent data, it seems appropriate to screen patients with established CVD for subclinical hypothyroidism, as risk factor or worse prognosis. However, evidence from observational studies should be carefully evaluated until corroboration with randomized controlled studies. In analogy to screening, treatment of subclinical hypothyroidism is a source of controversies. Based on epidemiological studies suggesting higher risks, thyroid replacement therapy should be considered for TSH ≥10.0 mIU/L and might be considered for those with TSH between 7.0–9.9 mIU/L. As the risk is not increased for the majority of patients with TSH between 4.5 and 7.0 mIU/L, the benefit of thyroid replacement therapy would be probably less pronounced. However, an argument for therapy at low TSH threshold is to prevent early the progression to overt hypothyroidism. A counterargument is a chronic and probably life-long daily therapy in asymptomatic individuals requiring frequent medical controls. In the assessment of risks and benefits, overtreatment is an issue in the elderly with potential adverse events, such as atrial fibrillation or osteoporosis with hip fracture (20,21).
In this area of uncertainties and lack of evidence, the TRUST (Thyroid hormone Replacement for Untreated older adults with Subclinical Hypothyroidism a randomized controlled Trial among older adults), supported by the FP-7 EU funding (Specific Program Cooperation—Theme Health, Proposal No: 278148-2, NCT01660126), is currently assessing the impact of thyroid replacement therapy on quality of life, potential symptoms, cardiovascular risk factors, and biomarkers. In addition, further studies should investigate the impact of thyroid replacement of cardiovascular imaging endpoints, such as echocardiography (22). Echocardiography is the most used diagnostic tool for the diagnosis and risk stratification of heart disease, such as the measurement of left ventricular ejection fraction for systolic function, and assessment of Doppler transmitral pulsed-wave flow pattern, such as isovolumic relaxation time, a wave and E/A ratio, or tissue Doppler for the E/é ratio, for the diastolic function (23,24). Recently, other methods using three-dimensional echocardiography and longitudinal strain improved the detection of subclinical abnormalities with higher accuracy (25). Such mechanistic trial will provide strong evidence regarding the potential benefit of thyroid replacement therapy in term of HF endpoints. Regarding atherosclerosis, surrogate endpoints, such as carotid intima media thickness, computed tomography with the estimation of calcium score or intravascular ultrasound (IVUS) or optical coherence tomography (OCT) of coronary arteries. Given that thyroid replacement is not an emergent target for pharmaceutical companies, academics should initiate such clinical trials in order to strengthen evidence and address a condition that concern 10% of the elderly population (22).
Acknowledgements
Funding: N Rodondi has received funding for a randomized controlled trial on subclinical hypothyroidism (Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism: a randomised placebo-controlled, TRUST study) from the European Commission FP7-HEALTH-2011, Specific Programme “Cooperation”-Theme “Health” Investigator-driven clinical trials for therapeutic interventions in elderly population (Proposal No. 278148-2).
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Yue Liu (Associate professor, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012;379:1142-54. [Crossref] [PubMed]
- Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526-34. [Crossref] [PubMed]
- Díez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab 2004;89:4890-7. [Crossref] [PubMed]
- Polikar R, Burger AG, Scherrer U, et al. The thyroid and the heart. Circulation 1993;87:1435-41. [Crossref] [PubMed]
- Biondi B, Fazio S, Palmieri EA, et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 1999;84:2064-7. [Crossref] [PubMed]
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008;52:1152-9. [Crossref] [PubMed]
- Biondi B, Palmieri EA, Lombardi G, et al. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med 2002;137:904-14. [Crossref] [PubMed]
- Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol 2012;167:609-18. [Crossref] [PubMed]
- Rotondi M, Magri F, Chiovato L. Risk of coronary heart disease and mortality for adults with subclinical hypothyroidism. JAMA 2010;304:2481; author reply 2482. [Crossref] [PubMed]
- Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012;126:1040-9. [Crossref] [PubMed]
- Ochs N, Auer R, Bauer DC, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 2008;148:832-45. [Crossref] [PubMed]
- Simmonds MC, Higgins JP, Stewart LA, et al. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials 2005;2:209-17. [Crossref] [PubMed]
- Zhang M, Sara JD, Matsuzawa Y, et al. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2015;68:1125. [Crossref] [PubMed]
- Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012;172:811-7. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-94. [Crossref] [PubMed]
- Writing Committee Members, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [Crossref] [PubMed]
- LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;162:641-50. [Crossref] [PubMed]
- Blum MR, Bauer DC, Collet TH, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 2015;313:2055-65. [Crossref] [PubMed]
- Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 2012;172:799-809. [Crossref] [PubMed]
- Gencer B, Rodondi N. Should we screen for hypothyroidism in patients with cardiovascular disease? Eur Heart J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Patel MR, White RD, Abbara S, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol 2013;61:2207-31. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165-93. [Crossref] [PubMed]
- Bax JJ, Delgado V, Achenbach S, et al. Multimodality imaging: bird's eye view from The European Society of Cardiology Congress 2014 Barcelona, August 30-September 3, 2014. J Nucl Cardiol 2014;21:1245-51. [Crossref] [PubMed]