
Preoperative detection of pleural adhesions using dynamic chest radiography: prospective analysis
Highlight box
Key findings
• DCR had high specificity and negative predictive value for detecting pleural adhesions preoperatively.
What is known and what is new?
• DCR is a new modality that can dynamically capture X-rays.
• DCR is also useful for detecting pleural adhesions preoperatively.
What is the implication, and what should change now?
• DCR has the potential to become a common preoperative examination for detecting pleural adhesions.
Introduction
Dynamic chest radiography (DCR) is a flat-panel detector-based functional X-ray imaging modality that clarifies pulmonary ventilation, diaphragm movement, and lung circulation. As a feature, DCR can be performed with a conventional X-ray generator and does not require contrast medium, and the patient dose is <1.9 mGy, which is the dose limit for 2-directional chest radiography (front and side) recommended (1,2) by the International Atomic Energy Agency (IAEA). While there are some reports of its application for evaluating diaphragm movement (3) or air trapping in patients with chronic obstructive pulmonary disease (4) and for diagnosing pulmonary hypertension (5), there are still few reports written by surgeons concerning the application of DCR to thoracic surgery (6,7).
When planning a surgical approach and predicting operative time or bleeding volume, it is important to determine the presence of pleural adhesions before surgery. We have been using DCR to detect pleural adhesions for preoperative patients with thoracic disease.
We therefore described the preoperative DCR and intra-operating findings, evaluated the consistency of preoperative detection for pleural adhesions, and now report its utility. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1226/rc).
Methods
Patients
This prospective study was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (Approval No: 20-18). The informed consent from each patient was obtained before examinations and the contents of this study was also disclosed at our hospital. The subjects of this study were continuous all of patients who underwent DCR before surgery from January 2020 to May 2022, excluding patients with pneumothorax or mesothelioma, as these diseases are characterized by spaces or adhesions in the thoracic cavity. In all patients, DCR was performed within three days before surgery.
Imaging protocol of DCR
The imaging protocol is shown in Figure 1. DCR was performed during respiration using a conventional radiography system (RADSpeed Pro; SHIMADZU, Kyoto, Japan) and a flat-panel detector (AeroDR fine; Konica Minolta, Tokyo, Japan). The patients were instructed to inhale and exhale after tidal breathing. The X-ray exposure conditions were as follows: tube voltage, 100 kV; tube current, 80 mA; pulse duration of pulsed X-ray, 6.3 ms; source-to-image distance, 2 m; additional filter, 2.8 mm Al + 0.1 mm Cu for filtering out soft X-rays. The exposure time was approximately 14 seconds. The pixel size was 200×200 µm, the matrix size was 4,248×4,248, and the overall image area was 43.2 cm × 43.2 cm. The gray-level range of the images was 16 bits, and the signal intensity was proportional to the incident exposure of the X-ray detector. The dynamic image data, captured at 15 frames/second, were synchronized with pulsed X-ray. Using pulsed X-ray prevented excessive radiation exposure to the subjects. The entrance surface dose was approximately 1.7 mGy.
Image analyses
DCR provides several dynamic images. It was analyzed using the software program (Konica Minolta Inc., Tokyo, Japan) installed in an independent workstation (Operating system: Windows 10 Enterprise; Microsoft, Redmond, WA, USA; CPU: Intel® CoreTM i5-7500, 3.40 GHz; random access memory, 8 GB). Examples of DCR and its analysis are shown in Figure 2 (corresponding video materials are available in Video S1).
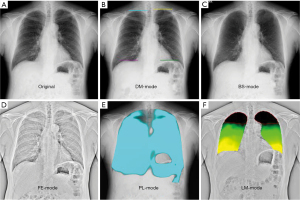
Original DCR (Figure 2A) is a simple dynamic image, DM-mode (Figure 2B) emphasizes the movement of the diaphragm, BS-mode (Figure 2C) has the clavicle and ribs erased, and FE-mode (Figure 2D) enhances the visibility of the lung marking. PL-mode (Figure 2E) and LM-mode (Figure 2F) concern ventilation. PL-mode shows the dynamic ventilation image, demonstrating a blue shadow in the position where the lung tissue is expanded by breathing. LM-mode visualizes upward movement of the lungs during expiration. The colorless area shows where there is little movement, we defined it as a low-motion area (LMA). In the other hands, green shows where there is moderate movement, and yellow shows where there is high movement.
The preoperative evaluation of pleural adhesions using DCR
The preoperative evaluations were performed by two thoracic surgeons with more than 10 years of thoracic surgery experience and having the certificate of specialty. PL-mode was referenced initially. If a blue shadow was found in all respiratory phases, the case was assessed as having no pleural adhesion. If the blue shadow disappeared in some areas of the lung field and pleural adhesion was suspected, the findings of FE-mode and LM-mode were added to PL-mode. In the FE-mode, the area of the field lacking a blue shadow in the PL-mode was carefully observed in order to identify any decreased movement of the lung. In addition, in LM-mode, when a colorless area was large and irregular changes were noted in the shadow shape, like a downward convex, pleural adhesion was considered present.
Consistency between the preoperative evaluation and intra-operative findings of pleural adhesions
Pleural adhesion was defined as the that spreading to more than 20% of the thoracic cavity and/or taking more than 5 minutes to dissect. The time for dissection of adhesion was measured by watching the operation video after surgery. The operation video was assessed by the operating surgeon and the other one. According to this definition, the preoperative evaluation of the existence of pleural adhesions using DCR was determined to be accurate or inaccurate based on intra-operative findings in all patients.
Analyses of the LMA ratio in LM-mode
In LM-mode, the LMA ratio were calculated using the dedicated software program from Konica Minolta, Inc. LMA ratio is a percentage obtained by dividing the LMA (mm2) by the lung field area of the surgical side (mm2). The LMA was defined as an area of less than 1.5 mm of upward movement during expiration. A retrospective analysis was performed on the relationship between the LMA ratio and pleural adhesions.
Statistical analyses
Mann-Whitney U test was used for continuous variables. P values of <0.05 were considered statistically significant. All statistical analyses and ROC curve about the LMA ratio and pleural adhesions were performed using the StatView software program (SAS Institute Inc., Cary, NC, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (8).
Results
Patient characteristics
The characteristics of the 120 patients [male, n=76; female, n=44; median age, 71 (range, 21–88) years old] are shown in Table 1. The consents were obtained from the all patients during the observation period. The thoracic diseases for surgery were primary lung cancer (n=82), benign lung tumor (n=13), metastatic lung cancer (n=9), mediastinum tumor (n=6), vascular malformation (n=4), pleural tumor (n=3), and infectious lung diseases (n=3). Thirty-eight patients had emphysema, and 11 had interstitial lung disease. Lobectomy was performed for 71 patients, segmentectomy for 17 patients, wedge resection for 23 patients, and tumor resection for 9 patients. With regard to the surgical approach, thoracotomy was performed in 63 patients, multiport video-assisted thoracoscopic surgery (mVATS) in 28 patients, uniportal video-assisted thoracoscopic surgery (uVATS) in 12 patients, and robotic-assisted thoracoscopic surgery (RATS) in 17 patients.
Table 1
| Characteristics | Values |
|---|---|
| Patients | 120 |
| Sex, n (%) | |
| Male | 76 (63.3) |
| Female | 44 (36.7) |
| Age (years), median [range] | 71 [21–88] |
| Thoracic disease, n (%) | |
| Primary lung cancer | 82 (68.4) |
| Benign lung tumor | 13 (10.8) |
| Metastatic lung cancer | 9 (7.5) |
| Mediastinum tumor | 6 (5.0) |
| Vascular malformation | 4 (3.3) |
| Pleural tumor | 3 (2.5) |
| Infectious lung diseases | 3 (2.5) |
| Underlying pulmonary disease, n (%) | |
| Presence of emphysema | 38 (31.7) |
| Presence of interstitial lung disease | 11 (9.2) |
| Surgical procedure, n (%) | |
| Lobectomy | 71 (59.2) |
| Segmentectomy | 17 (14.2) |
| Wedge resection | 23 (19.1) |
| Tumor resection | 9 (7.5) |
| Surgical approach, n (%) | |
| Thoracotomy | 63 (52.5) |
| mVATS | 28 (23.3) |
| uVATS | 12 (10.0) |
| RATS | 17 (14.2) |
mVATS, multiport video-assisted thoracoscopic surgery; uVATS, uniportal video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery.
Findings of DCR and consistency of the preoperative evaluation for pleural adhesions
The details are shown in Table 2. DCR was performed properly for 119 (99.2%) patients. Only 1 (0.8%) patient showed failure of DCR, as his DCR data included an artifact due to the patient moving his body in the process of taking images.
Table 2
| Category | N (%) |
|---|---|
| Appropriate taking DCR | 119 (99.2) |
| Suspected pleural adhesions using DCR | |
| Yes | 27 (22.5) |
| No | 92 (76.7) |
| Undecidable† | 1 (0.8) |
| Pleural adhesion‡ | |
| Absence | 88 (73.9) |
| Presence | 31 (26.1) |
| Consistency of preoperative evaluation‡ | |
| Accurate | 101 (84.9) |
| Inaccurate | 18 (15.1) |
†, patient with artifacts on DCR; ‡, one undecidable case was excluded. DCR, dynamic chest radiography.
According to the findings of DCR, pleural adhesions were suspected in 27 (22.5%) patients, not suspected in 92 (76.7%) patients, and undecidable for 1 (0.8%) patient (the patient whose findings showed an artifact). Surgery was then performed, and pleural adhesions were absent in 88 (73.9%) patients and present in 31 (26.1%) patients. The preoperative evaluation was thus accurate in 101 (84.9%) patients and inaccurate in 18 (15.1%) patients. The sensitivity was 64.5%, the specificity 91.0%, the positive predictive value 74.1%, and the negative predictive value 88.0% (Figure 3A). And on the patients with emphysema (n=38), the sensitivity was 57.1%, the specificity 91.7%, the positive predictive value 80.0%, and the negative predictive value 78.6%.
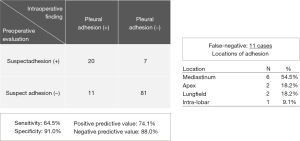
False-negatives (while no adhesion was suspected, it was present) were 11 cases, and the locations of the adhesions in these patients were the mediastinum (n=6; 3 in para-aorta, 2 in para-vertebra, and 1 in para-pericardium), apex (n=2), lung field (n=2), and inter-lobar (n=1) (Figure 3B).
Relationship between the LMA ratio and pleural adhesions
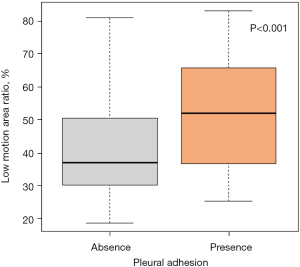
The median LMA ratio was 36.9% in patients without pleural adhesion and 52.0% in those with pleural adhesion. The LMA ratio was thus significantly higher in the patients with pleural adhesion than in those without it (P<0.001, Table 3 and Figure 4).
Table 3
| LM-mode | Pleural adhesion | P value | |
|---|---|---|---|
| Absence (n=88) | Presence (n=31) | ||
| Low-motion area ratio | 36.9%±13.0% | 52.0%±17.2% | <0.001 |
Data are presented as median ± standard deviation.
Case presentations
Four cases are shown in Figure 5.
- Case A was a patient with benign lung tumor. PL-mode showed a blue shadow in the whole left lung field, and there was considered to be no pleural adhesion. Pleural adhesion was ultimately not found (accurate).
- Case B was a patient with lung carcinoma. PL-mode showed the disappearance of the blue shadow in the right upper and middle areas of the lung field, and there was considered to be pleural adhesion. Pleural adhesions were extensively found between the right lung and chest wall (accurate).
- Case C was a patient with lung carcinoma. PL-mode showed a blue shadow in the whole left lung field, and there was considered to be no pleural adhesion. However, pleural adhesion in the left apex was ultimately found (inaccurate).
- Case D was a patient with non-tuberculous mycobacterial lung disease. PL-mode showed a blue shadow in almost the whole left lung field, and there was considered to be no pleural adhesion. However, pleural adhesions in the left posterior mediastinum around the para-aorta were ultimately found (inaccurate).
Discussion
DCR can provide information on several points, such as pulmonary ventilation, diaphragm movement, and lung circulation (9-11). It is expected to be clinically applied in various fields, and some reports have already been published, describing DCR as useful for evaluating chronic obstructive pulmonary disease and cystic fibrosis patients (1,2,12-14), in the clinical application of ventilation and perfusion (15,16), and for diagnosing pulmonary hypertension (5). However, there have been few reports of its surgical application, and the ones in existence are only case reports of surgery for pleural tumor (6).
In the preoperative evaluation of pleural invasion (7), pleural adhesion or invasion is sometimes evaluated with dynamic computed tomography (CT) (17-19) or magnetic resonance imaging (MRI) (20-22). However, these examinations are costly to perform and cannot be applied to all preoperative patients. One retrospective study written by a radiologist was reported concerning the evaluation of pleural adhesions with DCR (23). However, this study only used FE-mode to evaluate adhesion, and it was extremely subjective, with the findings difficult to evaluate without an expert. Furthermore, in that study, pleural adhesions were categorized by the adhesion grade based on the portion of the lung surface with adhesion. The authors therefore consider pleural adhesion to be a problem when it spreads extensively and thereby makes it difficult and time consuming to dissect such adhesions. Therefore, we used DCR with several different analytic modes in the present study (PL-mode, FE-mode, and LM-mode) for the preoperative detection of pleural adhesion, and adhesion was defined by the extension and dissecting time from a surgeon’s perspective.
Pleural adhesions make surgery difficult, and the operative time and bleeding volume can be increased compared to cases without adhesion. In addition, extensive pleural adhesions restrict the surgical approach, especially uVATS and RATS. In such cases, the surgical approach may have to be converted to mVATS or thoracotomy due to adhesions during an operation. For these reasons, it is important to determine the presence of pleural adhesions before surgery. And from this point of view as well, we conducted a prospective study on preoperative detection of pleural adhesions using DCR.
In the present study, DCR was able to be performed in patients of all ages (even for patients over 80 years old) with many different lung diseases, regardless of the presence of emphysema or interstitial lung disease. DCR was performed appropriate in 99.2% of cases, indicating that DCR is indeed very easy to perform for all preoperative patients. As a result, the utility of DCR for preoperatively detecting pleural adhesions was thus demonstrated. We determined DCR to have a high specificity and negative predictive value, and the results of the examination was equal to or better than that of dynamic CT or dynamic MRI (19,20). On the other hand, the utility of preoperative lung ultrasound is also reported useful to detect pleural adhesions (24-26). A systematic review by Shiroshita et al. showed very good results with the sensitivity of 70% and the specificity of 96% using ultrasound (24). Sasaki et al. reported the negative predictive value of 87.7% and the positive predictive value of 50.0% (25). Ultrasound is simple and does not expose to radiation, however, there are some areas where cannot be visualized due to obstructions such as the scapula and clavicle. In addition, ultrasound has the disadvantage that the individual visualization range is narrow compared to DCR which can see the entire lung filed. Although the interpretation of PL-mode is subjective and requires some familiarity and experience, the authors consider DCR to predict the absence of pleural adhesions with high probability, based on the present findings. However, there were some patients in whom the detection of adhesions was difficult. In particular, adhesions in the area of the mediastinum and apex were difficult to detect, as there areas show little movement compared to the outside of the lung field and around the diaphragm during breathing. DCR is a modality that captures movement of the lung, so these areas are its blind spots.
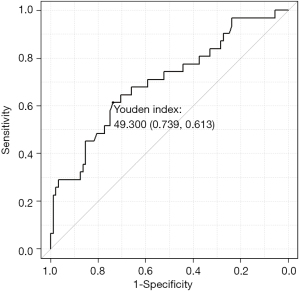
The LMA ratio is a supplemental parameter that compensates for these blind spots. It is an objective parameter, as the low motion of the lung can be captured as a numerical value. We found that a higher LMA ratio was significantly associated with an increased presence of pleural adhesions. According to the receiver operating characteristic curve (Figure 6), the cut-off is approximately 50%, so pleural adhesions could be suspected in a patient with an LMA ratio over 50%. However, the LMA ratio cannot be analyzed without using the software program developed by Konica Minolta, Inc. However, this software program is not yet available for use in clinical practice in Japanese hospitals. A software program that can conduct analyses in greater detail would likely improve the utility of DCR. We hope that, in the future, not only pleural adhesion but also invasion of the chest wall or around organs will be able to be detected easily using DCR.
The present study was associated with some limitations. First, this study was performed in a single center. Second, the DCR evaluation was subjective, being performed by the authors in the division of thoracic surgery. Finally, the LMA only increases the area as a result of pleural adhesions and does not accurately represent the location or degree of adhesions. However, we believe that the further accumulation of cases and updates to analytical software programs will eliminate these limitations.
Conclusions
We evaluated the consistency of the preoperative detection of pleural adhesions using DCR. To our knowledge, this is the first study conducted by thoracic surgeons to examine pleural adhesions using DCR in multiple patients before thoracic surgery. We demonstrated the utility of DCR, showing its high specificity and negative predictive value. Based on our findings, we hope that DCR will become a common preoperative examination for predicting the operative time or bleeding volume and deciding on the surgical approach, such as uVATS and RATS.
Acknowledgments
We thank the staff of the Radiology Department for their assistance in performing DCR and Konica Minolta, especially Noritsugu Matsutani, for performing the analyses in LM-mode.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1226/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1226/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1226/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1226/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective study was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (Approval No: 20-18). The informed consent from each patient was obtained before examinations and the contents of this study was also disclosed at our hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guidance levels of dose, dose rate and activity for medical exposure. In: Radiological protection for medical exposure to ionizing radiation. Safety guide. IAEA safety standards series No. SSG-46. Vienna: International Atomic Energy Agency (IAEA), 2018.
- International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. In: IAEA safety series No. 115. Vienna: International Atomic Energy Agency (IAEA), 1996.
- Hida T, Yamada Y, Ueyama M, et al. Decreased and slower diaphragmatic motion during forced breathing in severe COPD patients: Time-resolved quantitative analysis using dynamic chest radiography with a flat panel detector system. Eur J Radiol 2019;112:28-36. [Crossref] [PubMed]
- Ohkura N, Kasahara K, Watanabe S, et al. Dynamic-Ventilatory Digital Radiography in Air Flow Limitation: A Change in Lung Area Reflects Air Trapping. Respiration 2020;99:382-8. [Crossref] [PubMed]
- Yamasaki Y, Kamitani T, Abe K, et al. Diagnosis of Pulmonary Hypertension Using Dynamic Chest Radiography. Am J Respir Crit Care Med 2021;204:1336-7. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Saito D, et al. Case report: uniportal video-assisted thoracoscopic resection of a solitary fibrous tumor preoperatively predicted visceral pleura origin using dynamic chest radiography. J Cardiothorac Surg 2020;15:166. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Saito D, et al. Dynamic chest radiography: Novel and less-invasive imaging approach for preoperative assessments of pleural invasion and adhesion. Radiol Case Rep 2020;15:702-4. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Tanaka R. Dynamic chest radiography: flat-panel detector (FPD) based functional X-ray imaging. Radiol Phys Technol 2016;9:139-53. [Crossref] [PubMed]
- Hata A, Yamada Y, Tanaka R, et al. Dynamic Chest X-Ray Using a Flat-Panel Detector System: Technique and Applications. Korean J Radiol 2021;22:634-51. [Crossref] [PubMed]
- Tanaka R, Sanada S, Okazaki N, et al. Evaluation of pulmonary function using breathing chest radiography with a dynamic flat panel detector: primary results in pulmonary diseases. Invest Radiol 2006;41:735-45. [Crossref] [PubMed]
- FitzMaurice TS, McCann C, Nazareth DS, et al. Use of Dynamic Chest Radiography to Assess Treatment of Pulmonary Exacerbations in Cystic Fibrosis. Radiology 2022;303:675-81. [Crossref] [PubMed]
- FitzMaurice TS, McNamara PS, Nazareth D, et al. Utility and validity of dynamic chest radiography in cystic fibrosis (dynamic CF): an observational, non-controlled, non-randomised, single-centre, prospective study. BMJ Open Respir Res 2020;7:e000569. [Crossref] [PubMed]
- Ohkura N, Tanaka R, Watanabe S, et al. Chest Dynamic-Ventilatory Digital Radiography in Chronic Obstructive or Restrictive Lung Disease. Int J Chron Obstruct Pulmon Dis 2021;16:1393-9. [Crossref] [PubMed]
- Tanaka R, Matsumoto I, Tamura M, et al. Dynamic chest radiography: clinical validation of ventilation and perfusion metrics derived from changes in radiographic lung density compared to nuclear medicine imaging. Quant Imaging Med Surg 2021;11:4016-27. [Crossref] [PubMed]
- Ueyama M, Hashimoto S, Takeda A, et al. Prediction of forced vital capacity with dynamic chest radiography in interstitial lung disease. Eur J Radiol 2021;142:109866. [Crossref] [PubMed]
- Shirakawa T, Fukuda K, Miyamoto Y, et al. Parietal pleural invasion of lung masses: evaluation with CT performed during deep inspiration and expiration. Radiology 1994;192:809-11. [Crossref] [PubMed]
- Murata K, Takahashi M, Mori M, et al. Chest wall and mediastinal invasion by lung cancer: evaluation with multisection expiratory dynamic CT. Radiology 1994;191:251-5. [Crossref] [PubMed]
- Tokuno J, Shoji T, Sumitomo R, et al. Preoperative detection of pleural adhesions by respiratory dynamic computed tomography. World J Surg Oncol 2017;15:212. [Crossref] [PubMed]
- Akata S, Kajiwara N, Park J, et al. Evaluation of chest wall invasion by lung cancer using respiratory dynamic MRI. J Med Imaging Radiat Oncol 2008;52:36-9. [Crossref] [PubMed]
- Sakai S, Murayama S, Murakami J, et al. Bronchogenic carcinoma invasion of the chest wall: evaluation with dynamic cine MRI during breathing. J Comput Assist Tomogr 1997;21:595-600. [Crossref] [PubMed]
- Shiotani S, Sugimura K, Sugihara M, et al. Diagnosis of chest wall invasion by lung cancer: useful criteria for exclusion of the possibility of chest wall invasion with MR imaging. Radiat Med 2000;18:283-90. [PubMed]
- Tanaka R, Inoue D, Izumozaki A, et al. Preoperative evaluation of pleural adhesions with dynamic chest radiography: a retrospective study of 146 patients with lung cancer. Clin Radiol 2022;77:e689-96. [Crossref] [PubMed]
- Shiroshita A, Nakashima K, Takeshita M, et al. Preoperative Lung Ultrasound to Detect Pleural Adhesions: A Systematic Review and Meta-Analysis. Cureus 2021;13:e14866. [Crossref] [PubMed]
- Sasaki M, Kawabe M, Hirai S, et al. Preoperative detection of pleural adhesions by chest ultrasonography. Ann Thorac Surg 2005;80:439-42. [Crossref] [PubMed]
- Wei B, Wang T, Jiang F, et al. Use of transthoracic ultrasound to predict pleural adhesions: a prospective blinded study. Thorac Cardiovasc Surg 2012;60:101-4. [Crossref] [PubMed]




