
This article has an erratum available at: http://dx.doi.org/10.21037/jtd-2024-05 the article has been update on 2024-11-04 at here.
Influence of hepatic dysfunction in patients who underwent tricuspid valve surgery
Highlight box
Key findings
• The most suitable tool for predicting late mortality was the MELD-XI score, with a cutoff value of 13 points.
What is known and what is new?
• Hepatic dysfunction is a risk factor for cardiac surgery.
• Surgery for patients with severe tricuspid regurgitation can be performed with relatively low morbidity and operative mortality, regardless of associated hepatic dysfunction.
What is the implication, and what should change now?
• The MELD score significantly improved after surgery for hepatic dysfunction
• Even with favorable early outcomes, compromised long-term survival in patients with hepatic dysfunction suggests that early referral could lead to better outcomes in patients with severe tricuspid regurgitation.
Introduction
Hepatic dysfunction (HD) is a risk factor for cardiac surgery (1-4). Chronic tricuspid regurgitation (TR) is associated with HD (5), and in patients with chronic TR on HD, the patient’s liver function worsens if surgery is not performed. There are no treatment options for cardiac cirrhosis other than TR correction.
It is not sufficient to evaluate the risk of TR surgery based on results from decades ago (6,7), as recently reported results of TR surgery have significantly improved compared with those in the past (8,9). The risk of cardiac surgery in patients with HD, which has been investigated in many studies, is often based on data for primary cirrhosis, rather than cardiac cirrhosis (1,10-12), and although the Model for End-Stage Liver Disease (MELD) was initially designed to predict outcomes in patients with severe liver disease receiving transjugular intrahepatic portosystemic shunts, it was shown to be an effective predictor of survival (13).
Some studies have evaluated the MELD score in patients undergoing cardiac surgery (14,15); however, it is difficult to conclude whether it predicts morbidity and mortality after cardiac surgery (16). The value required to calculate the MELD score is often prolonged in patients taking warfarin due to atrial fibrillation, regardless of liver function. Therefore, we aimed to investigate the clinical impact of HD in patients undergoing tricuspid valve surgery (TV). Additionally, the risk assessment tool and cutoff value for the appropriate time to reduce late mortality after TR surgery were analyzed. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1741/rc).
Methods
Study population
In total, 159 patients underwent TV at our institution between October 2008 and July 2017. A retrospective review of consecutive patients diagnosed with moderate to severe TR before surgery was performed; 101 patients (mean age: 61.6±12.6 years; male: 34, female: 67) underwent TR surgery (Figure 1) and were included in the analysis. Fifty-eight patients who underwent TV surgery, but were not included in the study, showed mild tricuspid regurgitation. Three patients exhibited Ebstein’s anomaly, and one patient did not undergo TR surgery; this patient exhibited biventricular dysfunction with severe TR, and underwent cardiac transplantation after applying extracorporeal membrane oxygenation.
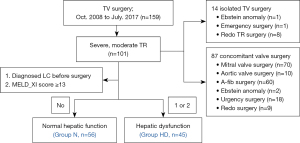
To examine the significance of HD in patients undergoing TR surgery, we divided patients into groups according to the presence of HD. HD was defined as clinically or radiologically diagnosed liver cirrhosis before surgery, or a preoperative MELD-XI score ≥13.
The MELD scoring system has been validated for the prediction of survival in patients with end-stage liver disease. A MELD score >13 was the cutoff for hospital mortality in patients who underwent cardiac surgery, as previously reported; however, patients with heart failure before TR surgery took anticoagulation drugs for various reasons, causing high MELD scores due to the increased prothrombin time international normalization ratio (PT-INR) associated with anticoagulation. To exclude the effects of anticoagulation on hepatic functional assessment, modified formulae, such as the MELD-XI and modified MELD, were introduced in the preoperative risk assessment.
The MELD scores were defined by the following equations:
- MELD score = 3.8* LN (total bilirubin) + 11.2*LN (INR) + 9.6*(creatinine) + 6.4
Where LN is the normal log and INR is the international normalized ratio.
- MELD-XI score = 5.11*LN (total bilirubin) + 11.76*LN (creatinine) + 9.44 (17)
If albumin is >4.1:
- MELD-albumin score = 11.2*LN (1) + 3.78 LN (total bilirubin) + 9.57* LN (creatinine) + 6.43
If albumin is <4.1:
- MELD-albumin score = 11.2*LN (1+(4.1-albumin) + 3.78 LN (total bilirubin) + 9.57* LN (creatinine) + 6.43 (14)
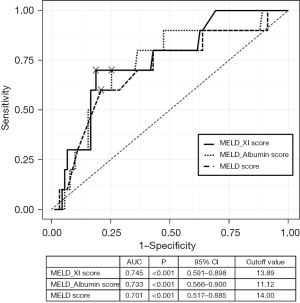
In our study, the MELD-XI score was the most accurate predictive risk assessment tool for TR surgery (Figure 2). The cutoff value for late mortality was 13 points for all scoring systems. In the analysis of the receiver operating characteristic curve for late mortality, the cutoff value for the MELD-XI score was 13.89; t HD was defined as a score of ≥13 to include all patients with HD in this study.
The primary outcomes were operative mortality (defined as mortality within <30 days), major complications, and length of intensive care unit (ICU) and postoperative hospital stays. Major complications were defined as reexploration for bleeding, neurological complications, low cardiac output syndrome, multiple organ failure, and pulmonary complications.
The secondary outcome was defined as the degree of change in HD after cardiac surgery. Changes in the MELD, MELD-XI, and MELD-albumin scores were compared in the HD group, and an analysis was conducted to identify an appropriate predictive risk assessment tool to determine the appropriate time for TR surgery. Univariate and multivariate risk factor analyses for late mortality were conducted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Pusan National University Yangsan Hospital (No. 05-2017-185) and individual consent for this retrospective analysis was waived.
Surgical technique
All 101 patients underwent TV surgery; isolated TR and combined mitral valve surgery were performed in 14 (13.9%) and 70 (69.3%) patients, respectively. The surgical approach comprised a median sternotomy (32.7%) or right mini-thoracotomy (67.3%), according to the surgeon’s preference and accompanying surgery. Surgical procedures included tricuspid valve replacement (10.9%) and tricuspid valvuloplasty (89.1%).
Conventional ascending aorta and bicaval cannulations for cardiopulmonary bypass were used in the full sternotomy approach. Right thoracotomy was performed with a 6 cm submammary incision, and the chest was opened through the fourth intercostal space for a minimally invasive right mini-thoracotomy. In this case, peripheral cannulation was performed through the right internal jugular vein, right femoral vein, and right femoral artery. In most cases of redo surgery, beating-heart surgery was performed via the right mini-thoracotomy approach without aortic cross-clamping, wherein an additional venous cannula was inserted into the internal jugular vein or right atrium directly through the thoracotomy site to block flow from the superior vena cava to the right atrium. The superior vena cava was snared with a vessel loop or packed with gauze.
Perioperative management
Diuretics and dobutamine are administered to patients before surgery to encourage aggressive diuresis and remove excessive body water. The right ventricle and right atrium were dilated, with increased TR volumes. Eventually, right ventricular systolic function failed, diastolic pressure raised, and the interventricular septum moved toward the left ventricle during diastole, which raised the pressure; this is called restriction-dilation syndrome (18,19). TR surgery is associated with high surgical mortality and morbidity when performed at this time; thus, preoperative management focuses on reducing the effects of restriction-dilation syndrome.
After surgery, the same protocols of extubation, transfusion, and ICU discharge were used in all patients. Diuretics were prescribed until the pitting edema resolved; when the diuretic requirement decreased, a postoperative echocardiogram was performed, and the patient’s discharge was planned.
Clinical follow-up
Perioperative data regarding surgery were prospectively collected, and clinical follow-up was performed by a heart team at our hospital. Patients underwent echocardiographic assessment 1 week after surgery, and annually or biennially thereafter without unexpected events. Data for evaluating hepatic function were obtained using blood tests performed at the outpatient clinic at 3, 6, and 12 months after surgery. Patients taking warfarin initially visited the outpatient clinic once a week before the dosage of warfarin was determined, and then every 3 months thereafter. During each outpatient visit, patients underwent chest radiography and blood tests.
Changes in MELD score
The MELD score was calculated based on the results of blood tests performed at the outpatient clinic immediately before the surgery, as well as 1 week, 3 months, and 1 year after surgery. For patients with preoperative anticoagulation, the lowest PT-INR—checked immediately before surgery, after vitamin K use or cessation of warfarin—was used to calculate the preoperative MELD score. Since neutralization by vitamin K is often insufficient, the MELD-albumin score and MELD-XI were also considered for analyzing changes in HD.
Statistical analysis
Categorical variables were presented as frequencies and percentages and compared using the Chi-squared and Fisher’s exact test. Continuous variables were expressed as means ± standard deviations or medians with interquartile ranges and were compared using the independent t test or Mann-Whitney U test. Wilcoxon signed rank test was used to compare the differences between the two time points with Bonferroni correction for adjusting the statistical inference of multiple comparisons. The Kaplan-Meier method with log-rank test and Cox proportional hazard regression analysis were applied to investigate the influence of HD on long-term survival. Univariate and multivariate logistic regression analyses were performed to identify the prognostic factors that are independently related to late mortality. Because the two groups were not randomly assigned in this patient population, potential confounding and selection biases were accounted for by developing a propensity score matching (PS), calculated using a multivariable logistic regression model in which age, sex, diabetes mellitus, atrial fibrillation, New York Heart Association (NYHA) functional class, previous cardiac surgery, left ventricular ejection fraction, creatinine level, TV repair, isolated TV operation, and mitral valve surgery were the independent variables. The inverse probability of treatment weighted method was employed over alternatives, such as propensity score matching, due to the priority of retaining sample size. Inverse probability treatment weighting (IPTW) was calculated from following formula: (1/PS) in HD group and [1/(1-PS)] in normal group. After IPTW, prognosis of patients in the two groups were examined using univariate analyses and multivariate analyses using the IPTW values of the two groups. Firth’s penalized maximum likelihood bias reduction method for logistic regression, which has been shown to provide a solution in case of monotone likelihood (nonconvergence of likelihood function) (20), was employed.
All statistical analyses were carried out using SPSS 26.0 IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp), MedCalc® Statistical Software version 20.009 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021), and R statistical software (version 4.2.1; R Foundation, Vienna, Austria, http://www.r-project.org/). P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
While the preoperative demographics such as age and comorbidity of both groups demonstrated no statistical difference, the data associated with HD were significantly worse in the HD group (Table 1). The patients in the HD group were older [61.50 (52.00–70.50) vs. 65.00 (53.50–73.00) years; P=0.406], and had lower hemoglobin levels (12.70±2.31 vs. 11.08±2.31 g/dL; P=0.001) and platelet count [175.00 (131.25–221.50) 1,000/mm3vs. 136.00 (108.50–193.00) 1,000/mm3; P=0.018] compared with patients in the N group. Relative to the N group, higher preoperative creatinine levels [0.90 (0.75–1.08) vs. 1.12 (0.82–1.55) mL/min; P=0.001] were observed in the HD group. Patients on HD had a longer duration of illness, higher frequency of atrial fibrillation, and more patients with NYHA class IV; additionally, patients in the HD group demonstrated higher Childs-Turcotte-Pugh and MELD scores, and higher estimated surgical mortality calculated using the EuroSCORE II [4.02 (2.52–8.77) vs. 6.50 (3.61–12.75); P<0.015] than the N group. Inferior vena cava plethora, hepatic reversal flow, ascites, and thrombocytopenia were more commonly observed in the HD group.
Table 1
| Overall (n=101) | |||
|---|---|---|---|
| Group N (n=56) | Group HD (n=45) | P value | |
| Age, years, median (IQR) | 61.50 (52.00–70.50) | 65.00 (53.50–73.00) | 0.4062 |
| Female sex, n (%) | 38 (67.9) | 29 (64.4) | 0.7183 |
| BSA, m2, median (IQR) | 1.56 (1.44–1.68) | 1.58 (1.40–1.71) | 0.8862 |
| HTN, n (%) | 24 (42.9) | 26 (57.8) | 0.1363 |
| DM, n (%) | 9 (16.1) | 13 (28.9) | 0.1213 |
| Atrial fibrillation, n (%) | 35 (62.5) | 37 (82.2) | 0.0293 |
| COPD, n (%) | 4 (7.1) | 8 (17.8) | 0.3203 |
| CRF on dialysis, n (%) | 0 (0.0) | 2 (4.4) | 0.1964 |
| Dyslipidemia, n (%) | 35 (62.5) | 21 (46.7) | 0.1123 |
| Stroke, n (%) | 12 (21.4) | 6 (13.3) | 0.2913 |
| NYHA functional class, n (%) | 0.0854 | ||
| I | 6 (10.7) | 1 (2.2) | |
| II | 19 (33.9) | 9 (20.0) | |
| III | 16 (28.6) | 15 (33.3) | |
| IV | 15 (26.8) | 20 (44.4) | |
| Urgency or emergency, n (%) | 9 (16.1) | 10 (22.2) | 0.5594 |
| Ebstein anomaly, n (%) | 1 (1.8) | 2 (4.4) | 0.5844 |
| Previous cardiac surgery, n (%) | 5 (8.9) | 12 (26.7) | 0.0183 |
| Ascites, n (%) | 4 (7.1) | 13 (28.9) | 0.0043 |
| Childs-Turcotte-Pugh score, median (IQR) | 5.00 (5.00–6.00) | 6.00 (5.00–7.50) | <0.0012 |
| MELD score, median (IQR) | 8.92 (7.87–9.69) | 15.52 (13.55–18.65) | <0.0012 |
| EuroSCORE II, median (IQR) | 4.02 (2.52–8.77) | 6.50 (3.61–12.75) | 0.0152 |
| Echocardiographic characteristics | |||
| LVEF, %, median (IQR) | 63.00 (56.00–67.00) | 62.00 (56.50–65.00) | 0.3892 |
| Severe mitral regurgitation, n (%) | 19 (33.9) | 14 (31.1) | 0.7643 |
| Trans TV systolic pressure gradient, mmHg, median (IQR) | 51.00 (42.00–74.00) | 49.00 (28.50–64.00) | 0.1292 |
| Left atrial diameter, mm, median (IQR) | 55.00 (49.25–60.00) | 55.75 (49.70–61.75) | 0.4232 |
| IVC plethora, n (%) | 27 (48.2) | 32 (71.1) | 0.0203 |
| Hepatic vein reversal flow, n (%) | 12 (21.4) | 20 (44.4) | 0.0133 |
| Preoperative laboratory finding | |||
| Hemoglobin level, g/dL (mean ± SD) | 12.70±2.31 | 11.08±2.31 | 0.0011 |
| Platelet count, 1,000/mm3, median (IQR) | 175.00 (131.25–221.50) | 136.00 (108.50–193.00) | 0.0182 |
| Albumin, g/dL, median (IQR) | 4.00 (3.80–4.38) | 3.90 (3.55–4.30) | 0.2582 |
| Creatinine, mg/dL, median (IQR) | 0.90 (0.75–1.08) | 1.12 (0.82–1.55) | 0.0011 |
| Bilirubin, mg/dL, median (IQR) | 1.00 (0.70–1.30) | 1.50 (1.10–2.10) | <0.0012 |
| Prothrombin time, median (IQR) | 1.12 (1.05–1.24) | 1.44 (1.22–1.69) | <0.0012 |
| BNP, pg/mL, median (IQR) | 281.00 (127.00–564.50) | 330.50 (194.50–702.75) | 0.2502 |
1, P values were derived from independent t-test. 2, P values were derived from Mann-Whitney’s U test. 3, P values were derived by chi-square test. 4, P values were derived from Fisher’s exact test. Shapiro-Wilk’s test was employed for test of normality assumption. HD, hepatic dysfunction; N, normal; BNP, B-type natriuretic peptide; BSA, body surface area; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; DM, diabetes mellitus; EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; HTN, hypertension; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; MELD, model for end-stage liver disease; NYHA, New York Heart Association; SD, standard deviation; TV, tricuspid valve; IQR, interquartile range.
Operative data
Most operative variables were similar between groups (Table 2); however, TV replacement tended to be more common in Group HD, owing to the severity of TV degeneration and cases of reoperation.
Table 2
| Overall (n=101) | |||
|---|---|---|---|
| Group N (n=56) | Group HD (n=45) | P value | |
| TV repair, n (%) | 53 (94.6) | 37 (82.2) | 0.0583 |
| Ring annuloplasty, n (%) | 42 (75.0) | 34 (75.6) | 0.9492 |
| Suture annuloplasty, n (%) | 14 (25.0) | 8 (17.8) | 0.3822 |
| TV replacement, n (%) | 3 (5.4) | 8 (17.8) | 0.0583 |
| Mechanical prosthesis | 2 (3.6) | 5 (11.1) | 0.2373 |
| Bioprosthesis | 1 (1.8) | 3 (6.7) | 0.3213 |
| Isolated TV operation, n (%) | 5 (8.9) | 9 (20.0) | 0.1092 |
| Concomitant surgery, n (%) | 51 (91.1) | 36 (80.0) | 0.1092 |
| Mitral valve surgery | 40 (71.4) | 30 (66.7) | 0.6062 |
| Surgical ablation | 30 (53.6) | 30 (66.7) | 0.1832 |
| CABG | 0 (0) | 2 (4.4) | 0.1963 |
| Surgical approaches, n (%) | |||
| Sternotomy | 15 (26.8) | 18 (40.0) | 0.1592 |
| MICS | 41 (73.2) | 27 (60.0) | |
| With ACC | 51 (91.1) | 32 (71.1) | 0.0092 |
| Without ACC | 5 (8.9) | 13 (28.9) | |
| CPB time, min, median (IQR) | 148.00 (116.50–192.50) | 146.00 (110.00–195.00) | 0.8381 |
| ACC time, min, median (IQR) | 109.00 (92.00–141.00) | 121.00 (99.50–154.75) | 0.1721 |
1, P values were derived from Mann-Whitney’s U test. 2, P values were derived by chi-square test. 3, P values were derived from Fisher’s exact test. Shapiro-Wilk’s test was employed for test of normality assumption. HD, hepatic dysfunction; N, normal; ACC, aortic cross clamp; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; MICS, minimally invasive cardiac surgery; TV, tricuspid valve.
A right minithoracotomy approach was used in 67.3% of patients and beating-heart TV surgery without cardioplegic arrest was performed in most isolated TR patients through a right mini-thoracotomy (85.7%). The incidence of beating-heart TV surgery was statistically higher in the HD group than in the N group, and cardiopulmonary bypass and aortic cross-clamp times were comparable between the groups.
Mitral valve surgery was performed simultaneously in 40 and 30 patients in the N (91.1%) and HD (80.0%) groups, respectively, while surgical ablation was performed in 30 patients in both the N (53.6%) and HD (66.7%) groups.
Early outcomes
Due to preoperative thrombocytopenia, anemia, and bleeding tendency due to HD (Table 1), the incidence of transfusions was higher, and reoperations for bleeding were more common in the HD group than in the N group. The duration of ICU and hospital stays were also significantly longer in the HD group, and prolonged ventilation, pulmonary complication rates, acute renal failure were higher in the HD group (Table 3). However, early mortality was 0% in the N group and 2.2% (n=1) in the HD group (P=0.446). The cause of early death was septic shock due to ventilator-associated pneumonia 26 days after the operation in the HD group.
Table 3
| Overall (n=101) | ||||
|---|---|---|---|---|
| Group N (n=56) | Group HD (n=45) | P value | P value4 | |
| Early mortality, n (%) | 0 (0.0) | 1 (2.2) | 0.4463 | 0.375 |
| Hospital stay (days), median (IQR) | 7.00 (5.25–11.75) | 9.00 (6.50–21.50) | 0.0251 | 0.005 |
| ICU stay (hours), median (IQR) | 27.50 (24.00–50.00) | 53.00 (26.50–141.00) | 0.0011 | 0.001 |
| Major complication, n (%) | ||||
| Acute renal failure | 0 (0.0) | 5 (11.1) | 0.0153 | 0.042 |
| Stroke | 0 (0.0) | 1 (2.2) | 0.4463 | 0.387 |
| LCOS | 17 (30.4) | 20 (44.4) | 0.1442 | 0.054 |
| Arrhythmia | 28 (50.0) | 19 (42.2) | 0.4362 | 0.561 |
| Pulmonary complication | 4 (7.1) | 11 (24.4) | 0.0152 | 0.014 |
| Prolonged ventilation | 3 (5.4) | 13 (28.9) | 0.0012 | 0.003 |
| Reoperation for bleeding | 1 (1.8) | 7 (15.6) | 0.0213 | 0.028 |
| Transfusion incidence, n (%) | 33 (58.9) | 38 (84.4) | 0.0052 | 0.003 |
| Chest tube drain (12 hr) (mL), median (IQR) | 375.50 (212.00–572.25) | 440.00 (284.00–825.00) | 0.1641 | 0.046 |
| On table extubation, n (%) | 18 (32.1) | 9 (20.0) | 0.1712 | 0.123 |
| Pace maker insertion, n (%) | 1 (1.8) | 3 (6.7) | 0.3213 | 0.320 |
| Follow-up duration (month), median (IQR) | 100.23 (74.87–128.62) | 72.23 (41.58–116.25) | 0.0151 | 0.013 |
Shapiro-Wilk’s test was employed for test of normality assumption. 1, P values were derived from Mann-Whitney’s U test. 2, P values were derived by chi-square test. 3, P values were derived from Fisher’s exact test. 4, P values were derived from IPTW-weighted logistic regression or linear regression analysis. Firth’s penalized maximum likelihood bias reduction method for logistic regression which has been shown to provide a solution in case of monotone likelihood (nonconvergence of likelihood function) was employed. HD, hepatic dysfunction; N, normal; ICU, intensive care unit; LCOS, low cardiac output syndrome; IQR, interquartile range.
Change in MELD score
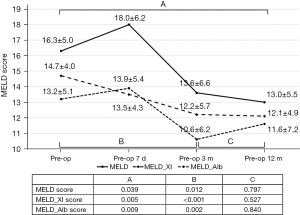
In the HD group, follow-up for the MELD score was completed up to 12 months postoperatively in 38 patients (84.4 %). Although 77.8% of the patients (35/45) took warfarin for anticoagulation due to atrial fibrillation or a mechanical mitral valve, 3- and 12-month postoperative MELD scores decreased significantly when compared with the preoperative MELD score (Figure 3); the MELD-XI and MELD-albumin scores demonstrated the same trend.
Mid-term follow up
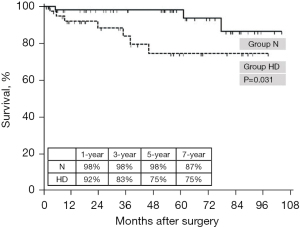
Follow-up was completed in 95 patients (94.1%), with a median follow-up duration of 43.7 months. During this period, 10 patients died (early mortality: 1, late mortality: 9), and none required readmission due to heart failure or reoperation for TR. Although a significant decrease in the MELD score and low early mortality were observed in the HD group, the 1-year (98% vs. 92%) and 3-year (98% vs. 83%) overall survival rates significantly decreased in the HD group (P=0.031; Figure 4).
In total, 10 deaths (one early and nine late) occurred during follow-up. A receiver operating characteristic curve was generated to determine the accuracy of the risk assessment tool that was most associated with late mortality. As shown in Figure 2, both the MELD-XI score (area under the curve: 0.745) and MELD-albumin score (area under the curve: 0.733) were significantly associated with late mortality; the best cutoff value was 13.89 points in the MELD-XI score.
Long-term follow up
When patients were followed for a longer period of time [100.23 (74.87–128.62) vs. 72.23 (41.58–116.25) years; P=0.013], 30 deaths occurred (12 (21.4%) vs. 18 (40.0); P=0.02). The 3-year (91% vs. 78%) and 5-year (91% vs. 71%) overall survival rates significantly decreased in the HD group (P=0.020; Figure 5). When performing risk analyses for long term survival, HD was identified as a stronger risk factor than age (Table 4). HD was found to be a potent risk factor for long-term survival, with a hazard ratio for HD of 1.965 [95% confidence interval (0.922, 4.186)]. Minimally invasive approach was found to have protective effect on late death, with a hazard ratio for MICS of 0.508 [95% confidence interval (0.244, 1.058)].
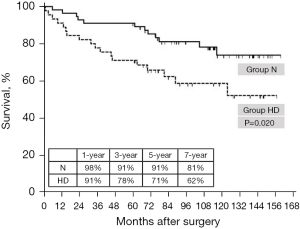
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value1 | P value2 | HR | 95% CI | P value1 | P value2 | ||
| Age | 1.073 | 1.036–1.111 | 0.000 | 0.000 | 1.069 | 1.033–1.106 | 0.000 | 0.000 | |
| Female, sex | 1.857 | 0.906–3.805 | 0.091 | 0.104 | |||||
| HTN | 2.547 | 1.188–5.459 | 0.016 | 0.010 | |||||
| DM | 1.187 | 0.509–2.769 | 0.692 | 0.752 | |||||
| Emergency | 1.383 | 0.593–3.223 | 0.453 | 0.446 | |||||
| A-fib | 2.362 | 0.902–6.187 | 0.080 | 0.053 | |||||
| EF | 1.008 | 0.969–1.048 | 0.695 | 0.789 | |||||
| TR peak PG | 0.992 | 0.977–1.006 | 0.272 | 0.256 | |||||
| Severe MR | 1.240 | 0.590–2.607 | 0.571 | 0.452 | |||||
| Severe TR | 2.550 | 1.133–5.739 | 0.024 | 0.026 | |||||
| Redo operation | 2.361 | 1.078–5.171 | 0.032 | 0.042 | |||||
| MICS | 0.505 | 0.246–1.036 | 0.062 | 0.059 | 0.508 | 0.244–1.058 | 0.070 | 0.010 | |
| Combine op. | 0.646 | 0.245–1.704 | 0.377 | 0.423 | |||||
| Euro SCORE II | 1.074 | 1.035–1.114 | 0.000 | 0.000 | |||||
| HD | 2.324 | 1.117–4.835 | 0.024 | 0.010 | 1.965 | 0.922–4.186 | 0.080 | 0.020 | |
| IVC plethora | 1.594 | 0.745–3.410 | 0.229 | 0.208 | |||||
1, crude P value using Cox regression analysis. 2, adjusted P value using IPTW-weighted Cox regression analysis. The variables that were significant at 0.10 alpha level in univariate analyses were included, and the multivariate model was created using a backward elimination method, and the probability was set at 0.10 for removal. A-fib, atrial fibrillation; DM, diabetes mellitus; EF, ejection fraction; EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; HD, hepatic dysfunction; HTN, hypertension; IVC, inferior vena cava; MICS, minimally invasive cardiac surgery; MR, mitral regurgitation; op., operation; TR, tricuspid regurgitation; PG, pressure gradient.
Discussion
This study evaluated the effect of HD in 101 patients who underwent TV surgery. Our findings revealed good overall early clinical outcomes, even in patients with HD. There was no significant difference in the initial mortality rate between patients with and without HD; however, there was a difference in late mortality. It was confirmed that the MELD score—which rose immediately after TR surgery—declined by 3–6 months, and remained suppressed until 12 months in the HD group. In our study, the MELD-XI score was the most predictive risk assessment tool for TR surgery; the cutoff value for lowering late mortality was 13.89 points.
HD is frequently associated with chronic TR, which increases surgical morbidity and mortality (1-3,5,14). Poor postoperative outcomes are related to the severity of TR and poor overall status of patients with right heart failure and HD (21). A previous study reported an operative mortality of 4–22.5% after TR surgery between 1974 and 2003 (7-9,14,22-26); however, the results of TR surgery have gradually improved since then (24,27,28). As described in this paper, the early results of TR surgery are improving; thus, we should not give up surgery by only considering the evaluated risk.
In our study, the mean MELD score in the HD group decreased significantly after surgery, and although 77.8% of patients were taking warfarin, the MELD score in the HD group decreased significantly 6 months after surgery. The MELD score declined by 6 months, and persisted until 12 months postoperatively (Figure 3).
Even though the current study showed favorable early results in patients with TR and HD, long-term survival was still significantly compromised, as shown by the Kaplan-Meier curve (Figures 4,5). Most patients exhibited improved hepatic function; however, some died after TR surgery. It is estimated that patients who died after TR surgery had already experienced irreversible liver damage; thus, liver function did not improve even if the TR was corrected. Since we do not know when irreversible damage of the liver is initiated, surgery for TR should be promptly performed before these irreversible changes occur; therefore, it is necessary to determine the optimal timing for TR surgery, and ensure that the intervention is not performed too late. In our study, the MELD-XI score was the most predictive risk assessment tool for TR surgery (Figure 2), with a cutoff value of 13.89 points for lowering late mortality. Patients with a MELD-XI score of ≥13 points exhibit poor late survival rates after TR surgery; therefore, it is desirable to perform surgery before reaching this stage of disease.
Even though TV surgery has been actively performed, TV surgery have historically been associated with high mortality and morbidity owing to complications because of various reasons, especially right-side heart failure (29). No robust prediction models currently exist to support clinical decision making for TV surgery. Recently, TriSCORE by Dreyfus (30) and Clinical Risk Score (31) have been proposed, but these studies were conducted with patients who underwent isolated t valve surgery; unlike our study, which focused on the effect of the patient’s HD after surgery. In the case of Clinical Risk Score, liver function was not included in the risk factor analysis, and in the case of Tri-Score, liver function was evaluated only through serum bilirubin.
Only one case of mortality was observed among the patients in this study. Minimally invasive TV surgery through the right mini-thoracotomy approach is known to be safe and feasible and may have contributed to these better results (9,32). Cardiac surgery through right mini-thoracotomy induces less bleeding, pain, and wound infection, and promotes faster recovery to daily life (33). Regarding beating-heart TV surgery without aortic cross-clamping, intrapericardial dissection is not required for the aortic cross-clamp site, or infusion of cardioplegic solution for cardiac arrest, which lowers cardiopulmonary bypass and operation time (34-36). Surgery without cardiac arrest may provide better protection of right ventricular function, and earlier detection of atrioventricular conduction block. Pre-operative optimization with inotropes and diuretics, as described in our methods, may have had a better effect on the patient's prognosis (37).
As the early mortality rate was low, a risk analysis was performed for long-term survival; however, there are insufficient data to make decisions regarding the cutoff value for TR surgery, and further research is required. The heart team’s approach is paramount during the preoperative evaluation and management of TR in patients with HD, and although patients with TR on HD exhibit better early outcomes than in the past, the timing of surgery has an impact on long-term survival. It should therefore be objectively evaluated whether TR surgery is beneficial before it is too late for surgery.
Limitations
This study has several limitations; first, we defined HD using the MELD-XI score. It is therefore difficult to separate the effects of renal failure, as the MELD score is directly dependent on renal function. Second, the sample size and follow-up duration may have been insufficient for obtaining accurate results. Third, this study lacks data on right-heart geometry and function due to the inherent problem of echo record in our center. Forth, we did not select patients suitable for surgery; cardiologists initially selected patients who were suitable for surgery and referred them to our department. Thus, there is a lack of data on all patients in our hospital.
Conclusions
Surgery for patients with severe TR can be performed with relatively low morbidity and operative mortality, regardless of associated HD. The MELD score improved significantly after TR surgery for HD; however, even with favorable early outcomes, compromised long-term survival in patients with HD suggests that early referral could lead to better outcomes in patients with severe TR. This also highlights the need for a risk assessment tool to determine the appropriate timing for surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1741/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1741/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1741/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Pusan National University Yangsan Hospital (No. 05-2017-185) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hayashida N, Shoujima T, Teshima H, et al. Clinical outcome after cardiac operations in patients with cirrhosis. Ann Thorac Surg 2004;77:500-5. [Crossref] [PubMed]
- Iino K, Takemura H. Cardiac surgery for patients with liver cirrhosis. Kyobu Geka 2017;70:596-600. [PubMed]
- Lin CH, Hsu RB. Cardiac surgery in patients with liver cirrhosis: risk factors for predicting mortality. World J Gastroenterol 2014;20:12608-14. [Crossref] [PubMed]
- Jacob KA, Hjortnaes J, Kranenburg G, et al. Mortality after cardiac surgery in patients with liver cirrhosis classified by the Child-Pugh score. Interact Cardiovasc Thorac Surg 2015;20:520-30. [Crossref] [PubMed]
- Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol 2002;90:1405-9. [Crossref] [PubMed]
- Filsoufi F, Anyanwu AC, Salzberg SP, et al. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg 2005;80:845-50. [Crossref] [PubMed]
- Ailawadi G, Lapar DJ, Swenson BR, et al. Model for end-stage liver disease predicts mortality for tricuspid valve surgery. Ann Thorac Surg 2009;87:1460-7; discussion 1467-8. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Santana O, et al. Tricuspid Valve Repair with Pericardial Tube Placement via a Right Minithoracotomy. J Heart Valve Dis 2015;24:338-41. [PubMed]
- Ricci D, Boffini M, Barbero C, et al. Minimally invasive tricuspid valve surgery in patients at high risk. J Thorac Cardiovasc Surg 2014;147:996-1001. [Crossref] [PubMed]
- An Y, Xiao YB, Zhong QJ. Open-heart surgery in patients with liver cirrhosis. Eur J Cardiothorac Surg 2007;31:1094-8. [Crossref] [PubMed]
- Filsoufi F, Salzberg SP, Rahmanian PB, et al. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl 2007;13:990-5. [Crossref] [PubMed]
- Wallwork K, Ali JM, Abu-Omar Y, et al. Does liver cirrhosis lead to inferior outcomes following cardiac surgery? Interact Cardiovasc Thorac Surg 2019;28:102-7. [Crossref] [PubMed]
- Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol 2013;3:50-60. [Crossref] [PubMed]
- Chokshi A, Cheema FH, Schaefle KJ, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant 2012;31:591-600. [Crossref] [PubMed]
- Suman A, Barnes DS, Zein NN, et al. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol 2004;2:719-23. [Crossref] [PubMed]
- Morisaki A, Hosono M, Sasaki Y, et al. Risk factor analysis in patients with liver cirrhosis undergoing cardiovascular operations. Ann Thorac Surg 2010;89:811-7. [Crossref] [PubMed]
- Abe S, Yoshihisa A, Takiguchi M, et al. Liver dysfunction assessed by model for end-stage liver disease excluding INR (MELD-XI) scoring system predicts adverse prognosis in heart failure. PLoS One 2014;9:e100618. [Crossref] [PubMed]
- Antunes MJ, Barlow JB. Management of tricuspid valve regurgitation. Heart 2007;93:271-6. [Crossref] [PubMed]
- Marquis-Gravel G, Bouchard D, Perrault LP, et al. Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis. Am Heart J 2012;163:851-858.e1. [Crossref] [PubMed]
- Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409-19. [Crossref] [PubMed]
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Yang JA, Kato TS, Shulman BP, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant 2012;31:601-10. [Crossref] [PubMed]
- Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672-8. [Crossref] [PubMed]
- Kim JB, Jung SH, Choo SJ, et al. Surgical outcomes of severe tricuspid regurgitation: predictors of adverse clinical outcomes. Heart 2013;99:181-7. [Crossref] [PubMed]
- Pfannmüller B, Moz M, Misfeld M, et al. Isolated tricuspid valve surgery in patients with previous cardiac surgery. J Thorac Cardiovasc Surg 2013;146:841-7. [Crossref] [PubMed]
- Maimaiti A, Wei L, Yang Y, et al. Benefits of a right anterolateral minithoracotomy rather than a median sternotomy in isolated tricuspid redo procedures. J Thorac Dis 2017;9:1281-8. [Crossref] [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [Crossref] [PubMed]
- Tsuda K, Koide M, Kunii Y, et al. Simplified model for end-stage liver disease score predicts mortality for tricuspid valve surgery. Interact Cardiovasc Thorac Surg 2013;16:630-5. [Crossref] [PubMed]
- Saran N, Dearani JA, Said SM, et al. Long-term outcomes of patients undergoing tricuspid valve surgery†. Eur J Cardiothorac Surg 2019;56:950-8. [Crossref] [PubMed]
- Dreyfus J, Audureau E, Bohbot Y, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J 2022;43:654-62. [Crossref] [PubMed]
- LaPar DJ, Likosky DS, Zhang M, et al. Development of a Risk Prediction Model and Clinical Risk Score for Isolated Tricuspid Valve Surgery. Ann Thorac Surg 2018;106:129-36. [Crossref] [PubMed]
- Pfannmueller B, Verevkin A, Borger MA, et al. Role of tricuspid valve repair for moderate tricuspid regurgitation during minimally invasive mitral valve surgery. Thorac Cardiovasc Surg 2013;61:386-91. [Crossref] [PubMed]
- Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: A systematic review and meta-analysis. Int J Cardiol 2016;223:554-60. [Crossref] [PubMed]
- Misfeld M, Davierwala P, Banusch J, et al. Minimally invasive, beating heart tricuspid valve surgery in a redo case. Ann Cardiothorac Surg 2017;6:290-3. [Crossref] [PubMed]
- Baraki H, Saito S, Al Ahmad A, et al. Beating Heart Versus Arrested Heart Isolated Tricuspid Valve Surgery. Int Heart J 2015;56:400-7. [Crossref] [PubMed]
- Salinas GE, Ramchandani M. Tricuspid valve replacement on a beating heart via a right minithoracotomy. Multimed Man Cardiothorac Surg 2013;2013:mmt006. [Crossref] [PubMed]
- Lee HS, Naqvi T. Tricuspid Valve Regurgitation Improvement with Medical Management in a Liver Transplant Candidate - Echocardiogram Guided Hemodynamics Understanding. Journal of the American College of Cardiology 2021;77:2884. [Crossref]


