
Long-term outcomes after coronary artery bypass graft with or without surgical ventricular reconstruction in patients with severe left ventricular dysfunction
Introduction
Although patients with chronic myocardial infarction (MI) and severe left ventricular (LV) dysfunction are at higher surgical risk, coronary artery bypass graft (CABG) is still the most widely applied technique to improve symptoms and prognosis in these patients (1-3). However, a small group of patients still develop LV dilatation after a maladaptive response to ischemic injury, leading to a spherically shaped LV and the formation of an aneurysm as well as the clinical syndrome of congestive heart failure (CHF). Surgical ventricular reconstruction (SVR) is a specific procedure developed for the management of CHF with LV remodeling caused by coronary artery disease (CAD) (4). Previous studies have demonstrated that this procedure restores the normal cardiac size and the elliptical shape of the heart. It has also been reported to reduce the LV volume and improve left ventricular ejection fraction (LVEF) and New York Heart Association (NYHA) class in these patients (5).
Although the benefit of SVR has been demonstrated, controversy remains as to whether CABG+SVR is superior to isolated CABG (I-CABG). Prucz et al. reported that SVR together with CABG might improve LV function to a greater degree than I-CABG and result in fewer rehospitalizations for CHF (6). However, the STICH trial concluded that despite CABG+SVR reducing the LV volume, this anatomical change was not associated with a greater improvement in symptoms or better survival (7). Prior et al. further reported that elective addition of SVR in patients undergoing CABG was not associated with a greater improvement in mortality (8). Although many previous studies sought to compare patients who underwent CABG and SVR to those who received I-CABG and different conclusions were drawn, only a few studies selected patients using a consistent imaging modality, and no studies provided a definitive answer about which kind of patients might benefit from SVR.
In recent years, contrast-enhanced cardiovascular magnetic resonance imaging (CE-CMR) has emerged as an accurate and non-invasive modality for the detection and quantification of myocardial scars and is commonly applied in the evaluation of patients with chronic MI and LV dysfunction to select appropriate treatment strategies. Therefore, it is the gold standard imaging modality for the assessment of not only LV function but also the transmurality of the scars (9). Based on the American Heart Association (AHA) 17-segmental model (10), our previous studies have demonstrated that the cardiac function of patients with >4 scar segments did not improve after I-CABG, and those with ≥6 scar segments experienced a higher risk of cardiovascular events (CVEs) post-I-CABG (11,12). However, the question remains as to whether SVR plus CABG can further improve the prognosis of patients with a considerable amount of myocardial scarring. Using this cohort of patients, we aimed to compare the outcomes of patients who underwent CABG and SVR to those who received I-CABG. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1214/rc).
Methods
Study population
This study included 140 consecutive patients with chronic MI and severe LV dysfunction clinically referred for CE-CMR [LV function and myocardial late gadolinium enhancement (LGE)] within 1 month before first-time surgery from April 2010 to June 2013. Patients were enrolled based on the following criteria: (I) CAD with >70% stenosis in two or more major vessels scheduled for surgery; (II) dyspnea as the predominant symptom; (III) previous Q-wave MI on electrocardiogram (ECG) and a history of MI ≥3 months before surgery; and (IV) LVEF of CMR ≤35% and at least two adjacent segments with wall motion abnormalities at rest, and the presence of anterior akinesia or dyskinesia of the LV on CMR. Patients were excluded if they had any of the following conditions: (I) any prior cardiac surgical interventions or concomitant surgical procedures (mitral/aortic valve repair or replacement); (II) hypertrophic obstructive cardiomyopathy or myocarditis; and (III) contraindications for CE-CMR examination (non-compatible biometallic implants, allergy to contrast agents, claustrophobia, etc.). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of Fuwai Hospital (No. 2010-259). All patients provided written informed consent.
An experienced surgical team was blinded to the patient identification and reviewed the CE-CMR of all subjects with LVEF ≤35% to determine their eligibility for SVR. During surgical exploration, the team was more inclined to perform SVR in cases with the presence of anterior/anteroseptal MI (scar tissue) and dominant anterior/anteroseptal akinesia or dyskinesia of the LV (6,13-15). Consequently, patients were divided into two groups according to the surgical strategy: those who underwent I-CABG (70 cases) and those who underwent CABG and SVR (70 cases). Transthoracic echocardiography (TTE) was performed preoperatively, before discharge, at 3, 6, and 12 months after surgery, and annually thereafter.
Surgical technique
All surgeries were performed by the same surgical team. CABG aimed to obtain complete revascularization, which was technically possible and performed in all patients. The left internal mammary artery (LIMA) and great saphenous vein (GSV) were harvested from each patient and the quality of grafts was assessed intra-operatively using a transit-time flow probe (TTFP; Medi-stim Butterfly flowmeter, Oslo, Norway). After surgery, all patients received standard pharmacotherapy for CAD.
In this study, cardioplegia was applied and I-CABG was performed under cardiac arrest. After aortic cross-clamping, cardiac arrest was accomplished via the antegrade administering of cold blood cardioplegia (CBC) (4 ℃) via the aortic root. Heart topical cooling was applied throughout the entire procedure. The CBC solution was a mixture of whole oxygenated blood and hyperkalemic solution at a 4:1 ratio. The initial induction dose was 15 to 20 mL/kg, and half of the initial dose was administered every 20 to 30 minutes. Additionally, the LIMA was anastomosed to the left anterior descending (LAD) branch, while the GSV was anastomosed to the obtuse marginal (OM) branch or posterior descending artery (PDA) branch.
For patients who underwent CABG and SVR, the SVR component was most commonly performed during a single period of cardioplegic arrest. The surgical indications for SVR included an anterior or anteroseptal MI (myocardial scar tissue), and the presence of dominant anterior akinesia or dyskinesia of the LV. In this procedure, after ventriculotomy is centered in the zone of anterior asynergy, a suture is placed in the interior of the ventricle to encircle the myocardial scar at the boundary between akinetic and viable tissue. The tightening of this suture brings the healthy myocardium together. The ventriculotomy defect was closed with a linear closure unless the incision was more than 2 to 3 cm, in which case a Dacron patch was used (16,17) (Figure S1 and Video 1). In our study, aneurysmectomy with linear repair was performed in 55 patients (78.6%), while circular reconstruction was performed in 15 patients (21.4%). After surgery, all patients received standard pharmacotherapy.
CMR protocol and imaging analysis
CMR was performed using a 1.5 Tesla scanner (Avanto, Siemens AG, Germany) according to a standardized scanning protocol. To evaluate the functional parameters, ECG-gated cine images were acquired with a steady-state free precession (SSFP) sequence in long-axis planes and contiguous short-axis slices from the atrioventricular ring to the apex as previously described (11). Ten to 15 minutes after intravenous injection of 0.2 mmol/kg of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) (gadopentetate dimeglumine, Magnevist, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA), LGE images were obtained using a phase-sensitive inversion-recovery gradient-echo pulse sequence in identical long-axis and short-axis planes.
The LGE evaluation and post-processing were performed using Argus software (Siemens AG, Munich, Germany). The cardiac function and LGE images were evaluated by two independent experienced radiologists (MJ Lu and SH Zhao) who were blinded to the clinical data using an identical 17-segment model. A five-point scale system was used to describe the transmural extent of LGE in each of the segments (scar score): 0 = no LGE, 1 = 1–25% LGE, 2 = 26–50% LGE, 3 = 51–75% LGE, and 4 = 76–100% LGE. If no agreement on the interpretations was reached, the image was reevaluated by two radiologists until a consensus was achieved. A cut-off value of 50% LGE was the optimal threshold to define segmental viability for predicting recovery of cardiac function (18). Additionally, the extent of scar tissue was quantified using the following definitions (18): (I) spatial extent, the number of affected segments; (II) normal segments, the number of segments with a scar score of 0; (III) viable segments, the number of segments with a scar score of 1 or 2; (IV) scar segments, the number of segments with a scar score of 3 or 4; and (V) total scar score (TSS), summation of the segmental scar scores for each patient. The severity of segmental wall motion was determined on a four-point scale system: 0 = normal, 1 = hypokinesis, 2 = akinesis, and 3 = dyskinesis. The wall motion score (WMS) was the summation of the WMSs for all 17 segments of the heart.
Outcomes and follow-up
The primary endpoint of the present study was a composite of CVEs defined as death from any cause, rehospitalization for CHF (a severe clinical syndrome characterized by the presence of dyspnea or limited exertion due to impaired cardiac ventricular filling or lowered cardiac contraction, leading to rehospitalization instead of staying at home), life-threatening ventricular arrhythmia (VA), non-fatal MI, and severe angina pectoris (AP) (11).
The subjects were followed up regularly for CVEs from the first day after discharge to the latest follow-up via telephone contact with the patients or their relatives, outpatient visit, or medical records review. All causes of death, CVEs, and functional status of the patients were recorded in detail.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) and SAS software (Version 9.4; SAS Institute Inc, Cary, NC). Continuous variables were presented as the mean ± standard deviation (SD) or median (interquartile range). Categorical variables were reported as absolute numbers and percentages. Between-group comparisons were performed using the Chi-squared test or Fisher’s exact test for categorical variables, and the Student’s t-test or Mann-Whitney U test for continuous variables that were normally and non-normally distributed, respectively. The improvement of LVEF and LV size as well as the effect of surgery were compared using a paired t-test. Survival curves were generated by the Kaplan-Meier method and compared by the log-rank test. All statistical tests were two-tailed, and P<0.05 was considered statistically significant.
Results
Study population
A total of 140 patients were enrolled and included in the final analysis (Figure 1). The baseline characteristics of the I-CABG group were well-matched to those of the CABG+SVR cohort, and no significant differences were observed between the two groups (Table 1). The mean age of patients in the I-CABG and CABG+SVR groups was 57.7±8.4 and 58.2±7.2 years, respectively (P=0.715). In addition, other baseline characteristics including hypertension, diabetes mellitus, and patients with NYHA class III/IV were similarly distributed between the two groups.
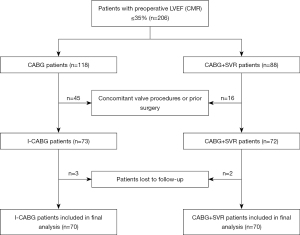
Table 1
| Variables | All patients (n=140) | I-CABG (n=70) | CABG+SVR (n=70) | P value |
|---|---|---|---|---|
| Age, years | 58.0±7.8 | 57.7±8.4 | 58.2±7.2 | 0.715 |
| Men | 124 (88.6) | 61 (87.1) | 63 (90.0) | 0.595 |
| Hypertension | 79 (56.4) | 38 (54.3) | 41 (58.6) | 0.609 |
| Diabetes mellitus | 57 (40.7) | 29 (41.4) | 28 (40.0) | 0.863 |
| Hypercholesterolemia | 76 (54.3) | 40 (57.1) | 36 (51.4) | 0.497 |
| COPD | 23 (16.4) | 12 (17.1) | 11 (15.7) | 0.820 |
| Stroke history | 13 (9.3) | 5 (7.1) | 8 (11.4) | 0.560 |
| Current smoker | 105 (75.0) | 51 (72.9) | 54 (77.1) | 0.558 |
| Family history of CAD | 59 (42.1) | 34 (48.6) | 25 (35.7) | 0.123 |
| Angiographic findings | 0.217 | |||
| Two-vessel lesions | 19 (13.6) | 7 (10.0) | 12 (17.1) | |
| Three-vessel lesions | 121 (86.4) | 63 (90.0) | 58 (82.9) | |
| NYHA class III/IV | 89 (63.6) | 42 (60.0) | 47 (67.1) | 0.380 |
| LVEF (Echo), % | 36.7±8.0 | 37.5±7.5 | 35.9±8.4 | 0.223 |
| Mitral grade | ||||
| Mild | 63 (30.7) | 36 (51.4) | 27 (38.6) | 0.198 |
| Moderate | 20 (14.3) | 7 (10.0) | 13 (18.6) | 0.198 |
| Euroscore | 6.0±2.4 | 5.8±2.2 | 6.2±2.5 | 0.238 |
Values are expressed as mean ± SD or n (%). CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; Echo, echocardiography; I-CABG, isolated coronary artery bypass graft; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation; SVR, surgical ventricular reconstruction.
Cardiac surgery
The CABG+SVR group had significantly longer operation time and CPB time than the I-CABG group (P=0.021 and P=0.002, respectively). The duration of aortic cross-clamping and the ventilation time were also longer for patients undergoing CABG and SVR (P=0.008 and P=0.019). The intensive care unit (ICU) duration, hospital stay, and number of grafts per patient were not significantly different between the two groups (P=0.064, P=0.340, and P=0.060, respectively). The procedural characteristics and outcomes of surgery are shown in Table 2.
Table 2
| Variables | All patients (n=140) | I-CABG (n=70) | CABG+SVR (n=70) | P value |
|---|---|---|---|---|
| Operation time, min | 233.0±49.6 | 223.4±46.8 | 242.6±50.7 | 0.021 |
| CPB time, min | 108.1±30.9 | 100.2±23.8 | 116.0±35.0 | 0.002 |
| Cross clamp, min | 70.3±19.8 | 65.9±17.8 | 74.8±20.8 | 0.008 |
| Ventilation time, h | 20.0 (16.0, 28.7) | 20.0 (15.0, 24.0) | 22.0 (17.0, 37.0) | 0.019 |
| ICU duration, h | 71.0 (46.0, 110.0) | 45.0 (69.0, 93.0) | 82.5 (55.3, 117.8) | 0.064 |
| Hospital stay, days | 10.5±4.2 | 10.2±5.3 | 10.8±2.7 | 0.340 |
| Grafts per patient | 3.3±0.8 | 3.4±0.8 | 3.1±0.7 | 0.060 |
| CABG outcomes | ||||
| New-onset AF | 12 (8.6) | 5 (7.1) | 7 (10.0) | 0.546 |
| Reoperation for bleeding | 5 (3.6) | 2 (2.9) | 3 (4.3) | 1.000 |
| Perioperative MI | 7 (5.0) | 3 (4.3) | 4 (5.7) | 1.000 |
| Stroke | 3 (2.1) | 2 (2.9) | 1 (1.4) | 1.000 |
| VA | 7 (5.0) | 3 (4.3) | 4 (5.7) | 1.000 |
| Renal failure requiring dialysis | 7 (5.0) | 4 (5.7) | 3 (4.3) | 1.000 |
| LCOS | 12 (8.6) | 7 (10.0) | 5 (7.1) | 0.546 |
| Death | 3 (2.1) | 2 (2.9) | 1 (1.4) | 1.000 |
Values are expressed as mean ± SD, median (interquartile range) or n (%). AF, atrial fibrillation; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; I-CABG, isolated coronary artery bypass graft; ICU, intensive care unit; LCOS, low cardiac output syndrome; MI, myocardial infarction; SD, standard deviation; SVR, surgical ventricular reconstruction; VA, ventricular arrhythmia.
In-hospital mortality was 2.9% in the I-CABG group (two patients) compared with 1.4% (one patient) in the CABG+SVR group (P=1.000). In the I-CABG group, there were two in-hospital mortalities: one owing to an exacerbation of CHF and the other owing to multiple organ dysfunction syndrome (MODS). In addition, the only one in-hospital mortality in the CABG+SVR group was attributable to MODS on postoperative day 9. The incidence of postoperative complications, including new-onset atrial fibrillation (AF), reoperation for bleeding, perioperative MI, stroke, life-threatening VA, renal failure requiring dialysis, and low cardiac output syndrome (LCOS), were similarly distributed among the two groups (Table 2).
CE-CMR results
No significant difference was observed between the I-CABG and CABG+SVR patients in terms of LV function or volume at baseline or LGE (including spatial extent, number of scar segments, viable segments, and TSS at baseline). The CMR results revealed more severe LV adverse remodeling in the CABG+SVR group, as evidenced by larger left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic volume index (LVESVI), but this was only marginally statistically significant (P=0.055 and P=0.079, respectively). The detailed CE-CMR results are summarized in Table 3. Moreover, the typical CE-CMRs of two patients at baseline and 6 months after I-CABG or CABG+SVR are shown in Figures S2,S3.
Table 3
| Baseline parameters | All patients (n=140) | I-CABG (n=70) | CABG+SVR (n=70) | P value |
|---|---|---|---|---|
| LVEF, % | 27.5±5.7 | 28.3±5.6 | 26.6±5.7 | 0.077 |
| LVEDD, mm | 62.7±7.2 | 61.5±6.5 | 63.8±7.7 | 0.055 |
| LVEDVI, mL/m2 | 113.4±31.3 | 109.4±28.7 | 117.3±33.4 | 0.135 |
| LVESVI, mL/m2 | 82.3±27.3 | 78.2±25.3 | 86.3±28.7 | 0.079 |
| CI, L/min/m2 | 2.2±0.7 | 2.2±0.6 | 2.2±0.8 | 0.998 |
| Dysfunctional segments | 15.5±2.0 | 15.2±2.2 | 15.8±1.8 | 0.097 |
| WMS | 24.7±4.6 | 24.0±5.1 | 25.4±4.0 | 0.051 |
| Myocardial LGE | ||||
| Spatial extent | 13.6±2.3 | 13.6±2.4 | 13.6±2.2 | 0.971 |
| Scar segments | 5.7±0.8 | 5.6±0.9 | 5.8±0.7 | 0.206 |
| Viable segments | 8.2±2.1 | 8.4±2.0 | 8.1±2.3 | 0.429 |
| Total scar score | 29.7±5.6 | 29.2±6.1 | 30.2±4.9 | 0.275 |
Values are expressed as mean ± SD. CABG, coronary artery bypass graft; CI, cardiac index; I-CABG, isolated coronary artery bypass graft; LGE, late gadolinium enhancement; LVEDD, left ventricular end-diastolic diameter; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; SD, standard deviation; SVR, surgical ventricular reconstruction; WMS, wall motion score.
Long-term outcomes
The average time for follow-up of all subjects was 123.1±12.7 (range, 102–140) months. Both groups showed a significant improvement in LVEF by TTE. The mean LVEF improved from 37.5%±7.5% to 45.4%±8.5% in the I-CABG group (P<0.001) compared with 35.9%±8.4% to 48.1%±8.9% in the CABG+SVR group (P<0.001) (Figure 2A). The change in LVEDD between 6 months and the latest follow-up in I-CABG patients was not significantly different, while that for patients who underwent CABG and SVR was significantly different (P=0.506 vs. P<0.001, respectively, Figure 2B). Likewise, the change of LVEF/LVEDD between baseline and follow-up in I-CABG patients was lower than that for CABG+SVR patients (P=0.002 and P<0.001, respectively, Table 4). In addition, CABG+SVR patients were more likely to have a ≥5% increase in LVEF during follow-up (84.1% vs. 60.3%, P=0.002, Table 4).
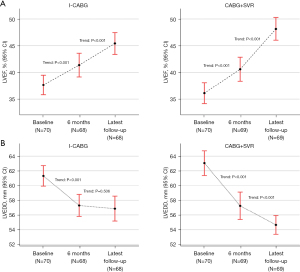
Table 4
| Outcomes | All patients (n=137) | I-CABG (n=68) | CABG+SVR (n=69) | P value |
|---|---|---|---|---|
| Rehospitalization for CHF | 16 (11.7) | 13 (19.1) | 3 (4.3) | 0.007 |
| VA | 4 (2.9) | 3 (4.4) | 1 (1.4) | 0.601 |
| Non-fatal MI | 3 (2.2) | 2 (2.9) | 1 (1.4) | 0.990 |
| Angina pectoris | 3 (2.2) | 1 (1.5) | 2 (2.9) | 1.000 |
| Deaths | 5 (3.6) | 3 (4.4) | 2 (2.9) | 0.987 |
| NYHA class I or II | 106 (77.4) | 47 (69.1) | 59 (85.5) | 0.030 |
| LVEDD change, % | −6.4±6.4 | −4.4±6.3 | −8.4±5.8 | <0.001 |
| LVEF change, % | 9.9±8.3 | 7.8±8.4 | 12.0±7.6 | 0.002 |
| LVEF improvement* | 99 (72.3) | 41 (60.3) | 58 (84.1) | 0.002 |
| No or trivial MR | 105 (76.6) | 47 (69.1) | 58 (84.1) | 0.115 |
*, LVEF improvement (Echo) was defined as ≥5% improvement in LVEF at follow-up. NYHA improvement was defined as any improvement in the NYHA class at follow-up. Values are expressed as mean ± SD or n (%). CABG, coronary artery bypass graft; CHF, congestive heart failure; Echo, echocardiography; I-CABG, isolated coronary artery bypass graft; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral valve regurgitation; NYHA, New York Heart Association; SD, standard deviation; SVR, surgical ventricular reconstruction; VA, ventricular arrhythmia.
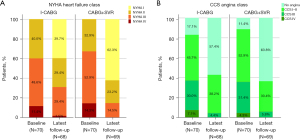
From baseline to the latest follow-up, the proportion of patients with NYHA class I increased, while that of patients with NYHA class III/IV decreased (Figure 3A). Likewise, the proportion of patients without severe AP increased, while that of patients with Canadian Cardiovascular Society (CCS) class III/IV decreased (Figure 3B). Moreover, the NYHA class improved significantly from 2.7±0.7 to 1.9±0.9 (P<0.001) in the I-CABG group and from 2.8±0.7 to 1.5±0.7 in the CABG+SVR group (P<0.001). At the latest follow-up, patients without angina were distributed similarly between these two groups (63.8% vs. 57.4%, P=0.616, Figure 3B). However, the CABG+SVR patients had a higher proportion of NYHA I/II class compared to the I-CABG patients (85.5% vs. 69.1%, P=0.030, Figure 3A).
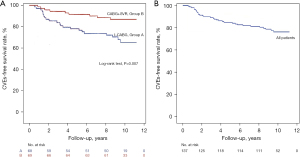
In our analysis, no significant difference between the two groups in preoperative mitral grade was observed. Sixty-three patients had mild mitral valve regurgitation (MR) (36 in the I-CABG group and 27 in the CABG+SVR group), while 20 patients had moderate MR (seven in the I-CABG group and 13 in the CABG+SVR group). In these 83 subjects during follow-up, MR was reduced in 61 (73.5%) patients compared with that before the operation. In addition, the I-CABG patients were more likely to be rehospitalized for CHF compared to the CABG+SVR patients (19.1% vs. 4.3%, P=0.007). Three deaths occurred in the I-CABG group, while two deaths occurred in the CABG+SVR group (4.4% vs. 2.9%, P=0.987). Patients who suffered VA, non-fatal MI, and AP recurrence were distributed similarly between the two groups (Table 4). Furthermore, the long-term cumulative CVE-free survival rate was significantly higher in the CABG+SVR patients (87.0% vs. 67.6%, P=0.007, Figure 4A), and that for patients overall in our study was 77.4% (Figure 4B).
Discussion
The salient findings of the present study were that in patients with a considerable amount of myocardial scarring (as detected by CE-CMR) and severe LV dysfunction, CABG+SVR provided a greater improvement in LVEF and NYHA class, and patients were less likely to be rehospitalized for CHF during follow-up compared to those who underwent I-CABG.
SVR is an effective treatment to improve LV function in patients with severe CHF and an LV anterior-apical aneurysm (19). Previous studies have shown that SVR not only reduces LVEDV and improves regional myocardial performance in non-ischemic areas, but also eliminates LV desynchrony and has a positive effect on patients’ long-term survival (5,20). Yet, it remains controversial whether these benefits are a result of the additional SVR or surgical revascularization. Howlett et al. reported that in patients with CHF and LV dysfunction undergoing I-CABG, the rates of cardiovascular hospitalization and CHF hospitalization were 45.2% and 25.6%, respectively (3). Klein et al. reported a 1-year survival rate of 90.6% in patients with previous MI and CHF (21). Prucz et al. demonstrated an excellent 4-year survival of 75% and 62% in CHF patients with ischemic cardiomyopathy who underwent CABG and SVR and I-CABG, respectively (6). In our patient cohort, the 10-year CVE-free survival rate for patients undergoing CABG and SVR was 87.0%. However, for patients who received I-CABG, this rate was only 67.6%, which was consistent with previous reports. For individuals whom we believed to be qualified for SVR yet did not receive it, Table 1 showed that these patients were similar to CABG+SVR patients in almost every baseline characteristic. Therefore, the differences in their operative protocol definitely affected their outcomes.
Numerous studies have demonstrated that preoperative LVESVI predicts survival in patients with ischemic cardiomyopathy (5,8,22). However, SVR may not be appropriate for many patients with LV enlargement and CHF, and we believe that SVR is only appropriate for a highly select group of patients who meet specific eligibility criteria. Current indications that are generally accepted for SVR involve anterior MI or aneurysm and LV akinesia or dyskinesia (23). These factors are not necessarily present in all ischemic cardiomyopathy patients with LV dilatation. Therefore, patients must be appropriately evaluated for a history of MI, LV enlargement, and most significantly, anterior wall nonviability (22). As a result, CE-CMR serves as the preferred modality and is used because it can provide accurate LV function and volume measurements and also determine the scar tissue (24). Castelvecchio et al. indicated that scar location affects long-term survival in CHF patients undergoing SVR (25), while Yamazaki et al. further demonstrated that accurate preoperative assessments of myocardial viability testing using CE-CMR are essential for better stratification of the SVR procedure (22). In the present study, all of the subjects had a considerable amount of scar tissue (≥5 scar segments), as evidenced by CE-CMR. No significant differences were found in the LGE parameters including scar segments, TSS, or LV size-related parameters at baseline between the two groups. The number of grafts in each group was also similar. Therefore, the two groups were comparable enough and we strongly believe that our results accurately reflect the discrepancies in follow-up outcomes between I-CABG and CABG+SVR.
Functional MR caused by ischemia is a common occurrence in individuals with a dilated ventricle. The longitudinal and transverse elongation of the LV causes lateral displacement and papillary muscle dysfunction, hindering leaflet coaptation (26). All individuals undergoing mitral valve procedures at the time of CABG were not included in the present study to ensure that the mitral valve operations were not confounding factors. In our analysis, MR was reduced in 61 (73.5%) of the patients during the follow-up compared with that before surgery, which indicates that both revascularization and SVR improve mitral valve function, and mitral repair or replacement may not be essential in patients with mild to moderate functional MR.
Our data also suggested that SVR can be safely performed in high-risk populations and is a useful strategy to treat chronic MI and severe LV dysfunction because of the differences in long-term prognosis and the percentage of LVEF increase. The idea that an appropriate SVR must be performed to achieve benefit is also supported by the current data. However, SVR is underutilized in real-world clinical situations, which might be attributable to the primary surgeon not appreciating the potential benefit, being unfamiliar with candidate selection, as well as a lack of training in the surgical technique. However, the number of patients with severe LV dysfunction and large aneurysms has been decreasing in developing countries like China in recent years. The patients in our cohort exhibited significant improvements not only in LVEF and LV sizes but also in NYHA class as well as clinical symptoms. We hope that our findings will increase awareness of SVR so that eligible patients will be referred to surgeons qualified to perform the SVR procedure in the future.
Limitations
Several limitations of this study merit attention. Firstly, despite the relatively long follow-up interval, the major limitation is that this is not a randomized controlled trial (RCT), but a single-center study with a relatively small cohort. Secondly, the classifications were based on a review of the preoperative imaging protocol and were thus inherently subjective. We tried to mitigate this subjectivity by having the same surgical team review all available CE-CMR protocols, TTEs, and ventriculograms. Also, despite having a specific indication of SVR, the final decision on whether to perform SVR is still made by the cardiac surgeon in charge of the procedure. As a result, selectivity bias indeed exists, which might also be a limitation of this study. Finally, CE-CMR was not performed in all patients at follow-up and the LV volume measurements for these patients were not reported. Thus, analyzing the results to detect the degree of revascularization and sufficient myocardial perfusion could not be performed. Nevertheless, all patients underwent TTE at 6 months instead of CE-CMR.
Conclusions
We conclude that in patients with chronic MI and severe LV dysfunction, CABG+SVR provided a greater improvement in terms of LVEF/LVEDD and NYHA class, and patients were less likely to be rehospitalized for CHF compared to those individuals who underwent I-CABG. Based on the 17-segmental model, patients with LV enlargement and a considerable amount of scar tissue (≥5 scar segments) located in the anterior segments will probably benefit from SVR in the long term.
Acknowledgments
We would like to gratefully thank the staff and faculty of the Department of Magnetic Resonance Imaging and Medical Research & Biometrics Center of Fuwai Hospital for their assistance in the design, data analysis, and implementation of this study.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81971588), the Beijing Natural Science Foundation (No. 7204289), and the Construction Research Project of the Key Laboratory (Cultivation) of Chinese Academy of Medical Sciences (No. 2019PT310025).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1214/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1214/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1214/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Velazquez EJ, Lee KL, Jones RH, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016;374:1511-20. [Crossref] [PubMed]
- Petrie MC, Jhund PS, She L, et al. Ten-Year Outcomes After Coronary Artery Bypass Grafting According to Age in Patients With Heart Failure and Left Ventricular Systolic Dysfunction: An Analysis of the Extended Follow-Up of the STICH Trial (Surgical Treatment for Ischemic Heart Failure). Circulation 2016;134:1314-24. [Crossref] [PubMed]
- Howlett JG, Stebbins A, Petrie MC, et al. CABG Improves Outcomes in Patients With Ischemic Cardiomyopathy: 10-Year Follow-Up of the STICH Trial. JACC Heart Fail 2019;7:878-87. [Crossref] [PubMed]
- Buckberg G, Athanasuleas C, Conte J. Surgical ventricular restoration for the treatment of heart failure. Nat Rev Cardiol 2012;9:703-16. [Crossref] [PubMed]
- Wakasa S, Matsui Y, Kobayashi J, et al. Estimating postoperative left ventricular volume: Identification of responders to surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2018;156:2088-2096.e3. [Crossref] [PubMed]
- Prucz RB, Weiss ES, Patel ND, et al. Coronary artery bypass grafting with or without surgical ventricular restoration: a comparison. Ann Thorac Surg 2008;86:806-14; discussion 806-14. [Crossref] [PubMed]
- Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705-17. [Crossref] [PubMed]
- Prior DL, Stevens SR, Holly TA, et al. Regional left ventricular function does not predict survival in ischaemic cardiomyopathy after cardiac surgery. Heart 2017;103:1359-67. [Crossref] [PubMed]
- Foley JR, Plein S, Greenwood JP. Assessment of stable coronary artery disease by cardiovascular magnetic resonance imaging: Current and emerging techniques. World J Cardiol 2017;9:92-108. [Crossref] [PubMed]
- American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462-508. [Crossref] [PubMed]
- Yang T, Lu M, Ouyang W, et al. Prognostic value of myocardial scar by magnetic resonance imaging in patients undergoing coronary artery bypass graft. Int J Cardiol 2021;326:49-54. [Crossref] [PubMed]
- Yang T, Lu MJ, Sun HS, et al. Myocardial scar identified by magnetic resonance imaging can predict left ventricular functional improvement after coronary artery bypass grafting. PLoS One 2013;8:e81991. [Crossref] [PubMed]
- Mickleborough LL, Merchant N, Ivanov J, et al. Left ventricular reconstruction: Early and late results. J Thorac Cardiovasc Surg 2004;128:27-37. [Crossref] [PubMed]
- Vanoverschelde JL, Depré C, Gerber BL, et al. Time course of functional recovery after coronary artery bypass graft surgery in patients with chronic left ventricular ischemic dysfunction. Am J Cardiol 2000;85:1432-9. [Crossref] [PubMed]
- Athanasuleas CL, Stanley AW Jr, Buckberg GD, et al. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. RESTORE group. Reconstructive Endoventricular Surgery, returning Torsion Original Radius Elliptical Shape to the LV. J Am Coll Cardiol 2001;37:1199-209. [Crossref] [PubMed]
- Cooley DA. Ventricular endoaneurysmorrhaphy: a simplified repair for extensive postinfarction aneurysm. J Card Surg 1989;4:200-5. [Crossref] [PubMed]
- Jatene AD. Left ventricular aneurysmectomy. Resection or reconstruction. J Thorac Cardiovasc Surg 1985;89:321-31. [Crossref] [PubMed]
- Kelle S, Roes SD, Klein C, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 2009;54:1770-7. [Crossref] [PubMed]
- Ferrell BE, Jimenez DC, Ahmad D, et al. Surgical ventricular reconstruction for ischemic cardiomyopathy-a systematic review and meta-analysis of 7,685 patients. Ann Cardiothorac Surg 2022;11:226-38. [Crossref] [PubMed]
- Gaudino M, Castelvecchio S, Rahouma M, et al. Long-term results of surgical ventricular reconstruction and comparison with the Surgical Treatment for Ischemic Heart Failure trial. J Thorac Cardiovasc Surg 2022;S0022-5223(22)00493-7.
- Klein P, Anker SD, Wechsler A, et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail 2019;21:1638-50. [Crossref] [PubMed]
- Yamazaki S, Doi K, Numata S, et al. Ventricular volume and myocardial viability, evaluated using cardiac magnetic resonance imaging, affect long-term results after surgical ventricular reconstruction. Eur J Cardiothorac Surg 2016;50:704-12. [Crossref] [PubMed]
- Rao V. Surgical ventricular remodeling: should we STICH or not? Curr Opin Cardiol 2017;32:744-7. [Crossref] [PubMed]
- Yamazaki S, Numata S, Inoue T, et al. Impact of right ventricular volume and function evaluated using cardiovascular magnetic resonance imaging on outcomes after surgical ventricular reconstruction. Eur J Cardiothorac Surg 2018;54:867-74. [Crossref] [PubMed]
- Castelvecchio S, Careri G, Ambrogi F, et al. Myocardial scar location as detected by cardiac magnetic resonance is associated with the outcome in heart failure patients undergoing surgical ventricular reconstruction. Eur J Cardiothorac Surg 2018;53:143-9. [Crossref] [PubMed]
- Song Y, Hu S, Sun H, et al. Results of Left Ventricular Reconstruction With and Without Mitral Valve Surgery. Ann Thorac Surg 2020;109:753-61. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


