
Prognostic effect of ground-glass opacity in subcentimeter invasive lung adenocarcinoma
Highlight box
Key findings
• Subcentimeter invasive lung adenocarcinoma is an emerging subgroup in clinical practice.
• The presence of a ground-glass opacity (GGO) component stratifies clinicopathologic characteristics and informs prognosis for these patients.
• Sublobar resection should be considered for subcentimeter IAC, even for those appearing as solid nodules; however, caution should be taken in the use of wedge resection.
What is known and what is new?
• Although subcentimeter lung nodules represent precursor lung cancer in most cases, there are still a few subcentimeter IACs.
• We investigated the clinicopathological features of subcentimeter IAC according to radiological appearance, determined the prognostic value of GGO component in this subgroup, and provided evidence on the proper clinical management for patients with subcentimeter nodules.
What is the implication, and what should change now?
• Subcentimeter lung nodules may present as IAC histologically, and sublobar resection is sufficient for those nodules. However, wedge resection should be used carefully.
Introduction
In recent decades, great advances have been made in radiological modalities, including thin-slice and low-dose computed tomography (CT). CT with high resolution and low radiation has revolutionized the field of lung cancer screening, making the detection of smaller lung nodules possible in a wider population (1). On account of their small size, subcentimeter adenocarcinomas (lesions 1.0 cm or smaller histologically) are often deemed less invasive and in early-stage malignancies. Many subcentimeter adenocarcinomas are histologically adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) in clinical practice. However, this is not always the case. Some subcentimeter adenocarcinomas have reported to demonstrate an invasive nature (2-5), the molecular mechanism behind which is still unknown. According to the eighth edition of the tumor, node, and metastasis (TNM) classification, patients with tumor not larger than 1 cm in diameter, as well as those with MIA, are designated as T1a and to be associated with favorable survival (6). In contrast, invasive adenocarcinomas (IAC) are associated with a risk of recurrence via lymphatic invasion, blood vessels, and the pleura (7), while those with AIS and MIA have a near 100% disease-specific survival. Conflicting factors affecting the prognosis of patients with subcentimeter IAC, including the small size and invasive nature, are controversial due to the small number of surgically managed patients. As demonstrated in many studies, the presence of ground-glass opacity (GGO) component has the ability to discriminate clinicopathological features and prognosis in patients with clinical stage IA lung adenocarcinoma (2,8,9). Few studies have been conducted focusing on the prognostic significance of the GGO component in subcentimeter IAC. In this study, we investigated the clinicopathological features of subcentimeter IAC based on radiological appearance, determined the prognostic value of GGO component in this subgroup, and provided evidence on the proper clinical management for patients with subcentimeter nodules. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1260/rc).
Methods
Study design
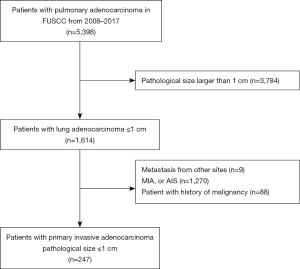
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board (IRB) of Fudan University Shanghai Cancer Center (IRB No. 2008223-9). Informed consents were waived due to the retrospective nature of the study. The data of patients with resected pulmonary adenocarcinoma admitted to the Department of Thoracic Surgery, FUSCC, Shanghai, China, from January 2008 to December 2017 were collected. Patients with the following features were excluded: (I) pathological tumor size larger than 1 cm, (II) metastases from other sites, (III) AIS or MIA, and (IV) a history of malignancy. A flowchart of patient enrollment in this study is shown in Figure 1. Data on clinicopathological variables were obtained by reviewing patient medical records. Data on age at diagnosis, gender, smoking history, CT appearance according to thin-section CT scan (TSCT), operative procedure, and prior history of malignancy were collected.
Radiological and histological evaluation
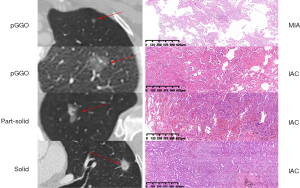
CT scans of the lung were performed with a helical technique using a 64 or 40 multi-detector scanner (Somatom Sensation, Siemens, Munich, Germany). We used the following scanning parameters: pitch, 1.2; section thickness and interval, 5.0 and 5.0 mm, respectively; reconstruction section width and interval, 1.0 and 1.0 mm, respectively; field of view (FOV), 375 mm; and tube voltage and reference milliampere per second, 120 kV and 270 mAs, respectively. CT scans were independently reviewed by 2 radiologists (S.W. and Y.J.) for radiological appearance. Tumor size on CT scan was defined as the maximum diameter of the lesion (ground-glass component included) on the axial plane. Consolidation-to-tumor ratio (CTR) was defined as the ratio of the maximum diameter of the solid component to the maximum diameter of the whole tumor on the TSCT scan on the axial plane (pure-GGO group: CTR =0; part-solid group: 0 < CTR < 1; solid group: CTR =1). Representative radiological figures of pure-GGO, part-solid GGO, and solid nodules are presented in Figure 2. For cases with different results, agreement was reached through discussion. Adenocarcinoma subtypes were classified based on the lung adenocarcinoma classification system released by the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society, and European Respiratory Society (7). Pathological tumor size was defined as the maximum diameter (lepidic component included) based on the maximum dimension of specimen. Pathological findings (including pathological tumor size, subtype, lymph node metastasis, visceral pleural invasion, and lymphovascular invasion) were reviewed by 2 experienced pathologists (Y.J. and Y.L.). Representative pathological figures of AIS/MIA and subcentimeter IAC are presented in Figure 2.
Surgical procedure
As mentioned in our previous study (10), intraoperative frozen section is applied to guide resection strategy for peripheral small-sized lung adenocarcinoma. Furthermore, size and location of the nodule and doctors’ individual preference all influence the final surgical procedure applied. In our institution, surgical procedure is determined comprehensively for each different patient.
Mutational analysis
Mutation analysis procedure was performed in a manner described in previous studies (11,12). Reverse transcriptase polymerase chain reaction (RT-PCR) primers were designed to cover mutation hotspot regions of epidermal growth factor receptor (EGFR) (exons 18, 19, 20, and 21). Sanger sequencing was used to analyze the PCR-amplified products.
Follow-up procedure
For the first 3 years after surgery, physical examination, ultrasonography of the abdominal/cervical/supraclavicular regions, chest CT scans, and magnetic resonance imaging of the brain were performed every 4 months. These tests were performed every 6 months in the third to the fifth year, and annually from then on. Bone scans were performed annually. Overall survival (OS) was considered to be the time between the day of surgery and the day of death or last follow-up. Deaths from other causes were considered as censored. OS was recorded based on prior telephone follow-up or clinic visit. Recurrence-free survival (RFS) was defined as the period from the day of surgery to the day of first recurrence or last follow-up. Patients who died from other causes were considered to be censored with no event for the calculation of RFS. Local recurrence was defined as recurrence in the ipsilateral thorax, including the resection margins of the lung or bronchus, hilar lymph nodes, mediastinal lymph nodes, and malignant pleural effusion.
Statistical analysis
Data were analyzed by using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). The Kaplan-Meier (KM) method was used to analyze OS and RFS, and the log-rank test was used to compare the differences between groups. Survival curves were generated with GraphPad Prism (version 8.01; GraphPad Software Inc., La Jolla, CA, US). All tests were 2-tailed, and statistical significance was set at a P value less than 0.05.
Results
Clinicopathological characteristics
A total of 247 patients with subcentimeter IAC were enrolled. Patients were divided into 3 groups according to radiological findings. There were 66 patients with pure GGOs (CTR =0), 107 patients with part-solid GGOs (0 < CTR < 1.0), and 74 patients with solid nodules (CTR =1.0) nodules. The clinical and histological characteristics are summarized in Tables 1,2. A greater proportion of female patients had pure GGO (P=0.086), yet the difference was not significant.
Table 1
| Patient characteristic | Total (n=247) | Pure GGO (n=66) | Part-solid GGO (n=107) | Solid (n=74) | P value |
|---|---|---|---|---|---|
| Gender, n (%) | 0.086 | ||||
| Male | 100 (40.5) | 21 (31.8) | 42 (39.3) | 37 (50.0) | |
| Female | 147 (59.5) | 45 (68.2) | 65 (60.7) | 37 (50.0) | |
| Respiratory risk, n (%) | 0.208 | ||||
| Active smoker | 73 (29.6) | 20 (30.3) | 26 (24.3) | 27 (36.5) | |
| Never smoker | 174 (70.4) | 46 (69.7) | 81 (75.7) | 47 (63.5) | |
| Age at surgery (years), median (range) | 58.4 (22–82) | 58.2 (38–82) | 57.8 (22–82) | 59.3 (25–79) | 0.594 |
| Radiological size (mm), average (Q1, Q3) | 12.6 (9, 15) | 11.6 (9, 12) | 14.4 (10, 16) | 12.4 (9, 14) | <0.001 |
| Radiological solid component (mm), average (Q1, Q3) | 7.1 (0, 6) | 0 (0, 0) | 7.7 (4, 10) | 12.4 (9, 14) | <0.001 |
| Accompanied with AIS/MIA lesions, n (%) | 0.635 | ||||
| Yes | 70 (28.3) | 20 (30.3) | 27 (25.2) | 23 (31.1) | |
| No | 177 (71.7) | 46 (69.7) | 80 (74.8) | 51 (68.9) |
GGO, ground-glass opacity; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Table 2
| Pathological characteristic | Total (n=247) | Pure GGO (n=66) | Part-solid GGO (n=107) | Solid (n=74) | P value |
|---|---|---|---|---|---|
| Pathological tumor size (mm), average (range) | 8.64 (4–10) | 8.73 (5–10) | 8.88 (5–10) | 8.23 (4–10) | 0.003 |
| Pathological invasive tumor size (mm), average (range) | 8.28 (4–10) | 8.14 (4–10) | 8.46 (4.5–10) | 8.13 (4–10) | 0.032 |
| Location, n (%) | |||||
| Right upper lobe | 68 (27.5) | 29 (43.9) | 23 (21.5) | 16 (21.6) | |
| Right middle lobe | 22 (8.9) | 5 (7.6) | 9 (8.4) | 8 (10.8) | |
| Right lower lobe | 36 (14.6) | 6 (9.1) | 15 (14.0) | 15 (20.3) | |
| Right upper + lower lobe | 15 (6.1) | 4 (6.1) | 10 (9.3) | 1 (1.4) | |
| Right upper + middle lobe | 4 (1.6) | 2 (3.0) | 2 (1.9) | 0 (0.0) | |
| Right upper + middle + lower lobe | 1 (0.4) | 0 (0.0) | 1 (0.9) | 0 (0.0) | |
| Left upper lobe | 52 (21.1) | 10 (15.2) | 24 (22.4) | 18 (24.3) | |
| Left lower lobe | 34 (13.8) | 7 (10.6) | 16 (15.0) | 11 (14.9) | |
| Left upper + lower lobe | 15 (6.1) | 3 (4.5) | 7 (6.5) | 5 (6.8) | |
| Range of resection, n (%) | 0.410 | ||||
| Lobectomy | 75 (30.4) | 16 (24.2) | 36 (33.6) | 23 (31.1) | |
| Segmectomy | 50 (20.2) | 13 (19.7) | 25 (23.4) | 12 (16.2) | |
| Wedge resection | 122 (49.4) | 37 (56.1) | 46 (43.0) | 39 (52.7) | |
| Histological subtype, n (%) | 0.081 | ||||
| Mucinous adenocarcinoma | 13 (5.3) | 1 (1.5) | 7 (6.5) | 5 (6.8) | |
| Adenocarcinoma | |||||
| Acinar | 193 (78.1) | 54 (81.8) | 86 (80.4) | 53 (71.6) | |
| Papillary | 24 (9.7) | 8 (12.1) | 10 (9.3) | 6 (8.1) | |
| Solid | 4 (1.6) | 0 (0.0) | 0 (0.0) | 4 (5.4) | |
| Micropapillary | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) | |
| Unidentified | 12 (4.9) | 3 (4.5) | 4 (3.7) | 5 (6.8) | |
| Lymph node metastasis (positive) | 6 (2.4) | 0 (0) | 1 (0.9) | 5 (6.8) | 0.003 |
| Number of lymph nodes sampling average (range) | 8.69 (0–33) | 6.60 (0–33) | 9.36 (0–32) | 9.58 (0–30) | 0.019 |
| Pleural invasion (positive) | 13 (5.3) | 2 (3.0) | 4 (3.7) | 7 (9.5) | 0.152 |
| Lymphovascular invasion (positive) | 4 (1.6) | 1 (1.5) | 1 (0.9) | 2 (2.7) | 0.649 |
GGO, ground-glass opacity.
Pathologically, greater diameter was seen in the pure-GGO and part-solid GGO group than in the solid group (mean 8.73 and 8.88 vs. 8.23; Table 2). The predominant histological subtype of IAC was analyzed. According to the proposed IASLC grading system, different pathologic subtypes were classified as follows: low grade (lepidic predominant), intermediate grade (acinar or papillary predominant), and high grade (solid or micropapillary predominant). Different distributions of the predominant histological subtype was observed in the 3 groups (P=0.081). High-grade adenocarcinoma subtypes were exclusively observed in the solid group, and groups with a GGO component had a significantly proportion of higher intermediate-grade subtypes.
Lymph node metastasis was observed in 6 patients and was significantly more frequent in the solid group (P=0.003). Lymph node metastasis, lymphovascular invasion, and pleural invasion were collectively referred to as pathological features associated with invasive behaviors. Binary logistic regression was performed to evaluate the predictive value of clinical features on invasive behaviors (Table 3). Radiological size [hazard ratio (HR) =1.106; 95% confidence interval (CI): 1.007–1.214; P=0.035) and presence of a GGO component (HR =0.247; 95% CI: 0.090–0.674; P=0.007) was significantly associated with invasive behaviors of subcentimeter IACs.
Table 3
| Logistic | OR (95% CI) | P |
|---|---|---|
| Gender (female vs. male) | 1.156 (0.270, 4.954) | 0.846 |
| Age | 1.019 (0.963, 1.080) | 0.509 |
| Smoking history (ever vs. never) | 2.545 (0.769, 8.418) | 0.126 |
| Number of lesions (multiple vs. solitary) | 1.743 (0.608, 4.998) | 0.302 |
| Radiological size | 1.106 (1.007, 1.214) | 0.035 |
| GGO component (presence vs. absence) | 0.247 (0.090, 0.674) | 0.007 |
OR, odds ratio; GGO, ground-glass opacity.
The median follow-up duration for the whole cohort was 61.9 months (interquartile range, 52.7–77.9 months). The median follow-up duration for patients who underwent lobectomy and sublobar resection was 63.9 months (interquartile range, 53.1–83.5 months) and 61.4 months (interquartile range, 52.9–86.5 months), respectively. The 2 groups were comparable.
Mutational status and distinct features
In all, 116 patients received testing for EGFR mutation, of whom 64 (55.2%) were found to harbor the EGFR mutation. The pure-GGO and part-solid GGO groups had a slightly higher ratio of patients with EGFR mutations than did the solid group (52.0% and 64.8% vs. 45.9%), yet the difference was not significant (P=0.184). Survival analysis regarding the mutational status of EGFR was performed, and there was no significant difference (Figure 3). The detailed mutation status of EGFR is listed in Table 4. We also noticed a considerable ratio of patients with subcentimeter IAC accompanied with other AIS/MIA lesions (Table 1). There were 70 (32.8%) patients with IAC accompanied with AIS/MIA lesions, and there were also no significant difference between the 3 groups in this regard (P=0.635). Survival analysis was also performed, and there were no significant difference in patients with subcentimeter IAC accompanied with other AIS/MIA lesions and patients with solitary lesions (Figure 3).
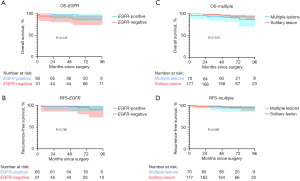
Table 4
| Mutational status, n (%) | Total (n=116) | Pure GGO (n=25) | Part-solid GGO (n=54) | Solid (n=37) | P value |
|---|---|---|---|---|---|
| EGFR_WT | 51 (44.0) | 12 (48.0) | 19 (35.2) | 20 (54.1) | 0.184 |
| EGFR_18 | 4 (3.4) | 0 (0.0) | 3 (5.6) | 1 (2.7) | 0.433 |
| EGFR_19 | 25 (21.6) | 3 (12.0) | 13 (24.1) | 9 (24.3) | 0.423 |
| EGFR_20 | 5 (4.3) | 1 (4.0) | 2 (3.7) | 2 (5.4) | 0.922 |
| EGFR_21 | 31 (26.7) | 9 (36.0) | 17 (31.5) | 5 (13.5) | 0.081 |
GGO, ground glass opacity; EGFR, epidermal growth factor receptor; WT, wild type.
Prognostic effect of GGO in subcentimeter IAC
Although possessing an invasive nature, patients with subcentimeter IAC has a favorable prognosis. OS and RFS based on radiological findings were demonstrated in Figure 4. Patients in the pure-GGO group and part-solid GGO group had a significantly better OS (5-year survival: 99.2% and 96.6% vs. 85.4%) and RFS (5-year survival: 100% and 95.5% vs. 90.1%) than did patients in the solid group.

Univariable and multivariable Cox regression analysis was also performed based on clinicopathological features (Tables 5,6). The presence of the GGO component was confirmed as an independent risk factor for a better RFS (HR =0.240; 95% CI: 0.071–0.813; P=0.022) and better OS (HR =0.293; 95% CI: 0.095–0.904; P=0.033).
Table 5
| Cox (RFS) | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender (male vs. female) | 1.727 (0.300, 9.936) | 0.541 | |||
| Age | 1.023 (0.948, 1.103) | 0.563 | |||
| Smoking history (ever vs. never) | 2.825 (0.526, 15.180) | 0.226 | |||
| Number of lesions (multiple vs. solitary) | 2.168 (0.557, 8.436) | 0.265 | |||
| Pathological size | 0.135 (0.003, 5.703) | 0.135 | |||
| Surgical procedure (segmentectomy vs. lobectomy) | 3.369 (0.169, 66.959) | 0.426 | |||
| Surgical procedure (wedge resection vs. lobectomy) | 9.662 (0.824, 113.253) | 0.071 | |||
| Lymph node metastasis (presence vs. absence) | 2.877 (0.235, 35.241) | 0.408 | |||
| Visceral pleural invasion (presence vs. absence) | 0.918 (0.094, 8.932) | 0.941 | |||
| Lymphovascular invasion (presence vs. absence) | 17.086 (1.356, 215.30) | 0.028 | 25.133 (2.101, 300.710) | 0.028 | |
| High grade predominant (presence vs. absence) | 0.000 (0.000, 0.000) | 0.985 | |||
| GGO component (presence vs. absence) | 0.233 (0.070, 0.778) | 0.018 | 0.240 (0.071, 0.813) | 0.022 | |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; GGO, ground-glass opacity.
Table 6
| Cox (OS) | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender (female vs. male) | 2.037 (0.450, 9.229) | 0.356 | |||
| Age | 1.048 (0.984, 1.116) | 0.148 | |||
| Smoking history (ever vs. never) | 1.410 (0.477, 4.169) | 0.001 | |||
| Number of lesions (multiple vs. solitary) | 1.746 (0.392, 7.780) | 0.081 | |||
| Pathological size | 0.092 (0.004, 2.194) | 0.141 | |||
| Surgical procedure (segmentectomy vs. lobectomy) | 1.209 (0.214, 6.833) | 0.830 | |||
| Surgical procedure (wedge resection vs. lobectomy) | 1.046 (0.305, 3.593) | 0.943 | |||
| Lymph node metastasis (presence vs. absence) | 6.808 (1.401, 33.074) | 0.017 | 6.994 (2.380, 20.557) | 0.010 | |
| Visceral pleural invasion (presence vs. absence) | 4.444 (1.290, 15.315) | 0.018 | 5.894 (1.528, 22.734) | <0.001 | |
| Lymphovascular invasion (presence vs. absence) | 0.000 (0.000, 0.000) | 0.983 | |||
| High grade predominant (presence vs. absence) | 2.575 (0.478, 13.875) | 0.271 | |||
| GGO component (presence vs. absence) | 0.296 (0.092, 0.953) | 0.041 | 0.293 (0.095, 0.904) | 0.033 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; GGO, ground-glass opacity.
Surgical procedures for subcentimeter IAC
OS and RFS regarding lobectomy and sublobar resection were also compared to determine the optimal surgical approach. Overall, lobectomy did not provide a significantly better RFS or OS in this cohort (Figure 5). After separating segmentectomy with wedge resection, we found that there was still no significant difference in OS among the 3 groups. However, patients who underwent wedge resection had significantly worse RFS than did those who underwent lobectomy (P=0.043). In univariable or multivariable Cox regression analyses, surgical approach (segmentectomy vs. lobectomy and wedge resection vs. lobectomy) was not an independent risk factor (Tables 5,6).

Due to the more invasive behavior observed in patients with solid lesions, we performed survival analysis in this subgroup (Figure 5). Although more extensive, lobectomy did not provide a significantly better RFS or OS (RFS: P=0.273; OS: P=0.857).
We found that patients in the pure GGO group underwent significantly less lymph node dissection (P=0.019). Average nodal yield for wedge, segmentectomy, and lobectomy was 4.96, 10.92, and 13.96, respectively (Table 2). A different surgical procedure implies a different extent of nodal harvest. In the pure-GGO group, more lobectomies than wedge resections were performed. We presume the difference in nodal harvest was due to the different surgical procedures applied.
Another concern related to less extensive surgical procedure is local relapse. Recurrence was observed in 12 patients in this cohort, of whom 4 experienced local relapse (Table 7). Although patients who underwent lobectomy had a lower rate of recurrence than did patients with sublobar resections, the disparity was not significant (P=0.159).
Table 7
| Surgical procedure | Local recurrence (n=4) | Distant recurrence (n=8) | No recurrence (n=235) | P |
|---|---|---|---|---|
| Lobectomy (n=75), n (%) | 1 (1.3) | 0 (0.0) | 74 (98.7) | 0.159 |
| Sublobar resection (n=172), n (%) | 3 (1.7) | 8 (4.7) | 161 (93.6) |
Discussion
For lung cancer patients after surgical treatment, TNM staging based on pathological information is essential for prognostic stratification. Although being a radiological parameter, GGO component has gradually been recognized as a significant prognostic factor in numerous studies (1,2,8,13). Our previous study (13) demonstrated the presence of GGO components to be a significant factor associated with better prognosis in patients with invasive stage I non-small cell lung cancer (NSCLC). In this study, we identified GGO component as a prognostic factor in a subgroup of patients with subcentimeter IACs.
As the simplest parameters that can be derived from images, lesion size is the most widely accepted criterion for radiological nodule evaluation. For pathological subcentimeter IACs, our study presented a larger average radiological size than pathology size in all 3 groups, with part-solid GGOs showing the highest deviation. Considering the differences in lung aeration and expansion, CT size tends to overestimate tumor size, especially in smaller adenocarcinomas (14-16). Therefore, as one of the most important predictors of outcome in lung cancer, pathological size should be carefully evaluated. As recommended by the IASLC, CT review should be performed to obtain a better impression of tumor size and relative proportion of invasive and lepidic components for part-solid GGO lesions (6,7).
Molecular pathology regarding subcentimeter IACs and GGO is an issue worth exploring. In this study, 56.4% patients harbored the EGFR mutation, which is consistent with our previous study (17) and other studies concerning Asia populations (18). We did not find there to be an association between EGFR mutation and subcentimeter IACs or GGO component. Studies have reported mixed results regarding the relationship between EGFR mutation and GGO component (19-21). Regarding invasiveness, Pi et al. found there to be no significant difference in EGFR mutation status in early versus advance stage adenocarcinoma in a Chinese cohort of 1,699 patients (22). Further studies with larger populations are required to determine whether molecular mutation is responsible for the invasiveness of subcentimeter adenocarcinomas.
There was a discrepancy in the prognostic factors for RFS and OS according to Cox regression analysis and KM survival analysis in this cohort. We speculate this difference lies in the relative high proportion of EGFR mutation in the Asian population (17). These patients may benefit from tyrosine kinase inhibitor treatment and achieve better prognosis regarding postoperative recurrence. For these patients, RFS is of greater importance than OS for analysis.
Radiological findings of ground-glass versus solid opacities tend to correspond respectively to the histological findings of lepidic versus invasive patterns. However, this correlation is not absolute (6). Heidinger et al. studied a subgroup of pure GGO patients and reported that 15.9% of patients ultimately had non-lepidic-predominant IAC (23). Additionally, in our previous study on a subgroup of stage I invasive NSCLC, 44.5% of patients in the pure-GGO had non-lepidic-predominant IAC. In this study, 88.3% of patients in the pure-GGO group had acinar-predominant adenocarcinoma. Radiological and pathological correlation between the ground-glass component and lepidic pattern requires further studies.
Although lobectomy has been the standard treatment for lung nodules suspected for lung cancer, the suitable surgical procedure for patients with small nodules remains controversial. Sublobar resection (anatomical segmentectomy or even wedge resection) is gaining favor due its ability to preserve more lung parenchyma and involve lower risk. For nodules with a predominant GGO component, consensus has been reached that sublobar resection is adequate (2,8,13,24), as the pathologic correlates of this appearance are mainly lepidic-predominant adenocarcinoma, MIA, or AIS (7). In this study, we found there to be no significant difference in the OS and RFS of patients who underwent lobectomy or sublobar resection despite the fact that patients in our cohort had an invasive histology. Therefore, it is clear that sublobar resection is adequate for patients with small GGOs even when histology is invasive.
The issue of solid nodule management is more controversial. For invasive tumor, sufficient surgical margins and adequate nodal evaluations are required for preventing locoregional recurrence and devising treatment and follow-up plans. However, our results showed that the subgroup of patients with subcentimeter solid IACs had no significant difference in RFS, OS, or local recurrence rate between patients underwent lobectomy versus those that went sublobar resection. These results are in line with the findings of the JCOG0802 trial (25), in which segmentectomy was compared with lobectomy in small-sized NSCLC. However, wedge resection was found to have a significantly worse RFS than lobectomy. This combination of findings provides some support for considering sublobar resection in small solid nodules, yet, caution should be taken when using wedge resection.
This study has several limitations. Although we present a large series for a rather rare subgroup, this study was single-center and retrospective in design, and there was potential for referral and selection bias. Moreover, the follow-up duration for estimating OS and RFS might not have been sufficiently long when the excellent prognosis of this cohort is considered.
Conclusions
Subcentimeter invasive lung adenocarcinoma is an emerging subgroup in clinical practice. The presence of a GGO component can stratify clinicopathologic characteristics and prognosis for patients in this subgroup. Sublobar resection should be considered for subcentimeter IACs even for those appearing as solid nodules; however, caution should be taken when using wedge resection.
Acknowledgments
This study was presented at the International Thoracic Surgical Oncology Summit 2022 of the American Association for Thoracic Surgery AATS as a poster.
Funding: This work was supported by the National Natural Science Foundation of People’s Republic of China (No. 81930073), the Shanghai Technology Innovation Action Project (No. 20JC1417200), Shanghai Municipal Key Clinical Specialty Project (No. SHSLCZDZK02104), and the Cooperation Project of Conquering Major Diseases in Xuhui District (No. XHLHGG202101).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1260/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1260/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1260/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the institutional review board of Fudan University Shanghai Cancer Center (IRB No. 2008223-9). Informed consents were waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y, Fu F, Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann Thorac Surg 2020;110:1796-804. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. [Crossref] [PubMed]
- Sakurai H, Nakagawa K, Watanabe S, et al. Clinicopathologic features of resected subcentimeter lung cancer. Ann Thorac Surg 2015;99:1731-8. [Crossref] [PubMed]
- Tsou KC, Hsu HH, Tsai TM, et al. Clinical outcome of subcentimeter non-small cell lung cancer after VATS resection: Single institute experience with 424 patients. J Formos Med Assoc 2020;119:399-405. [Crossref] [PubMed]
- Yao J, Zhu E, Li M, et al. Prognostic impact of micropapillary component in patients with node-negative subcentimeter lung adenocarcinoma: A Chinese cohort study. Thorac Cancer 2020;11:3566-75. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Hattori A, Hirayama S, Matsunaga T, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2019;14:265-75. [Crossref] [PubMed]
- Zhan Y, Peng X, Shan F, et al. Attenuation and Morphologic Characteristics Distinguishing a Ground-Glass Nodule Measuring 5-10 mm in Diameter as Invasive Lung Adenocarcinoma on Thin-Slice CT. AJR Am J Roentgenol 2019;213:W162-70. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Deng C, Zheng Q, Zhang Y, et al. Validation of the Novel International Association for the Study of Lung Cancer Grading System for Invasive Pulmonary Adenocarcinoma and Association With Common Driver Mutations. J Thorac Oncol 2021;16:1684-93. [Crossref] [PubMed]
- Pan Y, Zhang Y, Ye T, et al. Detection of Novel NRG1, EGFR, and MET Fusions in Lung Adenocarcinomas in the Chinese Population. J Thorac Oncol 2019;14:2003-8. [Crossref] [PubMed]
- Fu F, Zhang Y, Wen Z, et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J Thorac Oncol 2019;14:2133-42. [Crossref] [PubMed]
- Isaka T, Yokose T, Ito H, et al. Comparison between CT tumor size and pathological tumor size in frozen section examinations of lung adenocarcinoma. Lung Cancer 2014;85:40-6. [Crossref] [PubMed]
- Lampen-Sachar K, Zhao B, Zheng J, et al. Correlation between tumor measurement on Computed Tomography and resected specimen size in lung adenocarcinomas. Lung Cancer 2012;75:332-5. [Crossref] [PubMed]
- Sugawara H, Watanabe H, Kunimatsu A, et al. Tumor size in patients with severe pulmonary emphysema might be underestimated on preoperative CT. Eur Radiol 2022;32:163-73. [Crossref] [PubMed]
- Deng C, Zhang Y, Ma Z, et al. Prognostic value of epidermal growth factor receptor gene mutation in resected lung adenocarcinoma. J Thorac Cardiovasc Surg 2021;162:664-74.e7. [Crossref] [PubMed]
- Chen J, Yang H, Teo ASM, et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat Genet 2020;52:177-86. [Crossref] [PubMed]
- Hasegawa M, Sakai F, Ishikawa R, et al. CT Features of Epidermal Growth Factor Receptor-Mutated Adenocarcinoma of the Lung: Comparison with Nonmutated Adenocarcinoma. J Thorac Oncol 2016;11:819-26. [Crossref] [PubMed]
- Rizzo S, Petrella F, Buscarino V, et al. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol 2016;26:32-42. [Crossref] [PubMed]
- Yano M, Sasaki H, Kobayashi Y, et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006;1:413-6. [Crossref] [PubMed]
- Pi C, Xu CR, Zhang MF, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: Analysis based on large-scale data from China. Thorac Cancer 2018;9:814-9. [Crossref] [PubMed]
- Heidinger BH, Anderson KR, Nemec U, et al. Lung Adenocarcinoma Manifesting as Pure Ground-Glass Nodules: Correlating CT Size, Volume, Density, and Roundness with Histopathologic Invasion and Size. J Thorac Oncol 2017;12:1288-98. [Crossref] [PubMed]
- Yoshida J, Ishii G, Hishida T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: case selection based on high-resolution computed tomography-interim results. Jpn J Clin Oncol 2015;45:677-81. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
(English Language Editor: J. Gray)


