
Prognostic significance of lymph node dissection for lung cancer surgery: a narrative review
Introduction
Lymph node dissection in cancer surgery was first reported by Halsted et al. more than 120 years ago. They analyzed 50 cases of breast cancer surgery and reported that wide resection (including lymph node dissections and mastectomies) were performed on the basis of local recurrence (1).
As recommended by the European Society of Thoracic Surgeons (ESTS) guidelines (2) and the National Comprehensive Cancer Network (NCCN) guidelines (3), lymph node dissection was also applied to lung cancer and the standard surgical procedure for non-small cell lung cancer (NSCLC) became lobectomy or pneumonectomy with systematic lymph node dissection (SLND) (4-6). SLND has also been identified as important for postoperative survival and diagnostic staging (5,6). However, the prognostic significance of SLND is still controversial. For this study, we considered the lymph node dissection for lung cancer, including recent reports. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1527/rc).
Methods
By referring to past reports, we reviewed the process leading up to the establishment of SLND in lung cancer surgery. SLND was defined as a wide dissection from the upper mediastinum to the lower mediastinum regardless of the localization of the primary tumor. We compared five randomized prospective comparative studies on SLND and lymph node sampling (LNS) in lung cancer surgery. With reference to a recent report on segmentectomy, the prognostic significance of lymph node dissection was reconsidered. Based on past reports, immune responses in the regional lymph nodes targeted by SLND were also discussed (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Aug 4, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Lung cancer, lymph node dissection, systematic lymph node dissection, selective lymph node dissection, lymphadenectomy, and lymph node sampling |
| Timeframe | 1997–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: lymph node dissection for lung cancer |
| Exclusion criteria: research with similar conclusions | |
| Selection process | Ichiki conducted a literature search and analysis, consulted with all authors, and reached a consensus |
Lymph node dissection for lung cancer
Cahan et al. presented a radical pneumonectomy based on 39 successful cases. A radical pneumonectomy is defined as the excision of the lung in continuity with its regional lymph nodes located in the hilar and mediastinal areas (7). Lymph node dissection was applied to colon cancer, gastric cancer, and breast cancer, and its importance in lung cancer was recognized, after which the procedure was reported in detail. In addition, 48 cases of radical lobectomy were also reported (8). The extent of lymph node dissection in each lung lobe was proposed. Although the importance of lymph node dissection was firmly established, 41.5% of patients have hilar or mediastinal lymph nodes, which might be detected earlier by current imaging technology and detection sensitivities. Thus, there are limitations to directly applying it to current clinical practice. On the other hand, Sakaguchi et al. reported that bilateral mediastinal lymphadenectomy using median sternotomy were performed for left lung cancer or right upper lobe lung cancer to remove occult N3α (contralateral mediastinal lymph node) and N3γ (ipsilateral or contralateral supraclavicular/scalene muscle lymph node) lymph nodes. Further, if metastases were present in the highest mediastinum lymph nodes, a cervical lymph node dissection was also performed with an additional cervical collar incision, according to their analysis of lymph channels in 193 NSCLC cases (9).
SLND versus LNS
Izbicki et al. reported that SLND did not contribute to disease-free survival (DFS) or overall survival (OS) in surgical cases of NSCLC (10). In the subgroup analysis, there was a tendency (P=0.058) to prolong OS in pN1 or pN2 patients with SLND, although there was no significant difference.
Sugi et al. revealed that comparing SLND with LNS in patients with resected peripheral NSCLC with a tumor diameter of 2 cm or less showed no significant difference in recurrence rate and OS. However, the morbidity of SLND was significantly higher than LNS (26.8% vs. 3.4%) (11).
Darling et al. analyzed 1,023 cases with surgical N0 or N1 (less than hailar) NSCLC and compared SLND and LNS as the American College of Surgery Oncology Group (ACOSOG) Z0030 study. They found that there was no significant difference in OS and DFS between the two groups (12).
Allen et al. reported that the operative mortality of the ACOSOG Z0030 study was 0.76% for SLND and 2.0% for LNS and that the morbidity was 38% in each group. There was no significant difference between the two groups both in mortality and morbidity (13).
Wu et al. reported that in patients with resectable NSCLC, the median survival period was 59 months in the SLND group and 34 months in the LNS group. Further, the SLND group had significantly better OS than the LNS group (P=0.00000) (14).
Zhang et al. compared SLND and LNS (the mediastinal structures were not skeletonized) in resected cases of NSCLC. Although the SLND group was able to obtain significantly more lymph node stations than the LNS group (8.9 vs. 6.2, P<0.001), there was no difference in pathological staging. The SLND group had a significantly better 5-year survival rate than the LNS group (55.7% vs. 37.7%, P=0.005). LNS did not show a significant difference in OS between stage I and well-differentiated cancer cases, but SLND was significantly more effective in other cases (15). It was suggested that LNS should be considered only in limited cases such as stage I and well-differentiated cancer cases.
The results of the five randomized clinical trials are shown in Table 2. Two reported an improvement in OS with SLND, but the remaining 3 reported no significant difference between SLND and LNS. One study reported a significant increase in complications with SLND. Through these studies, the prognostic significance of lymph node dissection remained controversial. All studies except the ACOSOG Z0030 trial and data from Germany (10) were single center studies. Data from Germany (10) and China (14,15) included Stage III, whereas data from Japan (11) and ACOSOG Z0030 trial included only Stage I or II.
Table 2
| Trial | Country | Patients/study design/number of patients | Control arm | Median follow-up period (month) | 5-year survival | DFS | Morbidity | Mortality | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Izbicki | Germany | Operable NSCLC/RCT/N=169 | Lymph node sampling | 47.5 | 65.8% (54.8%) | Median DFS: 48 months (median DFS: 24 months) | NA | NA | 1998 | (10) |
| Sugi | Japan | Clinical N0 peripheral NSCLC less than 2 cm in diameter/RCT/N=115 | Lymph node sampling | 65 | 81.4% (83.9%) | NA | 26.8%* (3.4%) | 0% (0%) | 1998 | (11) |
| Wu | China | Clinical stage I-IIIA NSCLC/RCT/N=471 | Lymph node sampling | 43 | 48.37%* (36.98%) | NA | NA | 0.31% (0%) | 2002 | (14) |
| Daring Allen | America | Surgical N0 or N1 (less than hilar) NSCLC/RCT/N=1,023 | Lymph node sampling | 78 | MST: 8.5 years (MST: 8.1 years) | 5-year DFS: 68% (5-year DFS: 69%) | 0.76% (2.0%) | 38% (38%) | 2011 | (12,13) |
| Zhang | China | Clinical stage I-IIIA NSCLC/RCT/N=202 | Minimal mediastinal dissection (mediastinal structures were not skeletonized) | NA | 55.7%* (37.7%) | NA | 14.7% (14.0%) | 2.1% (1.9%) | 2013 | (15) |
Parentheses: data for control arm; *, P<0.05. DFS, disease-free survival; NSCLC, non-small cell lung cancer; RCT, randomized clinical trial; N, number; MST, median survival time; NA, not available.
Most studies, with the exception of the ACOSOG Z0030 data, had relatively small sample sizes and most studies found no significant difference in morbidity between the two groups. More frequent occult N2 disease were identified in SLND group than LNS group. Regarding survival outcomes, two Chinese data showed that the SLND group had significantly better survival than LNS group, whereas the other studies showed no difference. In the Wu trial, stage IIIA was more frequently enrolled in the SLND group. In the ACOSOG Z0030 trial, even in the LNS group, the quality of lymph node assessment was similar to that in the SLND group. From the outset, it made almost impossible to find a difference between the two groups. In addition, almost all studies had numerous biases in study design, including imprecise random sequence generation, imprecise allocation concealment, inherently impossible blinding, and imprecise intent-to-treat analyses.
Lobe-specific lymph node dissection (L-SLND)
Asamura et al. reported that subcarinal lymph node dissection is not always required for right upper and left upper tumors, because solitary metastases to the carina rarely occur (1.9% and 2.9%, respectively). It was reported that lymph node dissection of the lower mediastinal region including subcarinal lymph node can be avoided by conducting an intraoperative evaluation for primary lung cancer in the right upper lobe or right upper segment without metastasis in the hilar and upper mediastinal lymph nodes (16).
Okada et al. reported that upper mediastinal lymph node dissection should be performed for upper lobe lung cancer, but not for lower lobe lung cancer with negative hilar and subcarinal lymph nodes. They also indicated that subcarinal lymph node dissection may not be necessary for hilar and superior mediastinal node-negative upper lobe lung cancer (17). Additionally, they analyzed 735 patients with clinical surgical stage I NSCLC and compared L-SLND with SLND. L-SLND did not show a significant difference between SLND and L-SLND in DFS and OS (18).
The L-SLND has also gained wide acceptance as a deemed standard in recent years. If we look back, the radical lobectomy first advocated by Cahan et al. was a lymph node dissection that could be considered lobe-specific (8). Nohl et al. investigated 100 resected cases of lung cancer and demonstrated in detail the presence of lobe-specific lymphatic drainage channels (19).
Hishida et al. retrospectively investigated 5,392 cases of c-stage I or II NSCLC that underwent SLND or L-SLND in addition to lobectomy (20). L-SLND had a better prognosis than SLND (hazard ratio =0.68, 95% confidence interval: 0.60–0.77). Moreover, there was no difference in postoperative complications between them.
Adachi et al. retrospectively analyzed 565 cases of cT1a-T2b N0-1 M0 NSCLC who underwent lobectomy with LNS or L-SLND or SLND (21). In this analysis, there was no significant difference in the 5-year survival possibility between L-SLND and SLND after matching (73.5% and 75.3%, respectively. P=0.977). There was also no significant difference in the pN2 detection rate between the two groups (8.2% in both groups, P=0.779).
On the other hand, Maniwa et al. analyzed 335 patients with surgical N0 who underwent complete resection of NSCLC and reported that L-SLND significantly increased mediastinal lymph node recurrence when compared to SLND. There was no significant difference in DFS and OS between the two groups (22). The validity and usefulness of L-SLND has not yet been evaluated fully. However, a multicenter prospective study by the Japan Clinical Oncology Group (JCOG) 1413 comparing SLND and L-SLND is currently underway, and the results are awaited (23).
R uncertain [R(un)] resection
The International Association for the Study of Lung Cancer (IASLC) proposed a more detailed category ‘R(un)’ resections that negative margins but high risk of disease as shown below (5). (I) the intraoperative lymph node evaluation has less strict than SLND or L-SLND, (II) the highest mediastinal node removed is positive, (III) the bronchial resection margin shows carcinoma in situ, (IV) the pleural lavage cytology examination result is positive. Edwards et al. revealed that R factors have prognostic significance, with R(un) survival stratifying between R0 and R1 based on analysis of 14,712 resected lung cancer patients (24). The worse prognosis due to R-uncertainty may still have the potential to improve, especially with improved lymph node dissection.
Special aspects of lymph node dissection in segmentectomy
Saji et al. analyzed 1,106 peripheral NSCLC cases with tumor diameter ≤2 cm and consolidation-to-tumor ratio >0.5 on the computed tomography (CT) and compared segmentectomy to lobectomy. Though it was a study to analyze whether the prognosis of segmental resection was inferior to that of lobectomy, segmentectomy was significantly superior to lobectomy in OS. This research gave us an opportunity to reconsider the surgical method for peripheral small-sized lung cancer in the future. SLND or L-SLND was mandatory in this clinical trial. However, the proportion of SLND and L-SLND in each group seems to be unclear (25). Even if a segmentectomy was planned, they were converted to lobectomies in cases where the existence of lymph node metastasis was confirmed by intraoperative rapid histological diagnosis or in cases in which the surgical margin was insufficient.
Schlachtenberger et al. reported that 16.5% of patients with NSCLC ≤2 cm had lymph node upstage after surgery. It was emphasized that lymph node dissection and proper staging are important for resected NSCLC patients, regardless of tumor size or surgical approach (26).
Sublobar resection is expected to be applied more often in early NSCLC cases, but the confirmation of intraoperative lymph node metastasis and surgical margin will be essential in selecting sublobar resection. In segmentectomy, dissection of intersegmental lymph nodes tends to be negligent, and it seems necessary to examine the significance of lymph node dissection in segmentectomy.
Immunological effect after lymph node dissection
T cells are not sensitized in the tumor tissue itself, but in the lymph nodes closest to the lesion (the so-called regional lymph nodes). Cancer antigens released from cancer cells are captured by antigen-presenting cells (such as dendritic cells) and transported to regional lymph nodes by lymph flow. Naive T cells recognize cancer antigens presented by antigen-presenting cells in regional lymph nodes. They then activate and mature into effector T cells, after which they exhibit cancer-specific activity (Figure 1).
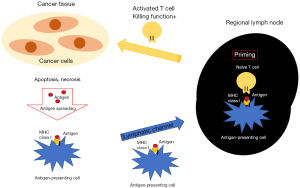
Theoretically, in order to completely remove cancer cells, it is necessary to remove the lymphatic channels with a sufficient resection margin. On the other hand, it is not yet clear how the removal of regional lymph nodes, which are the forefront of cancer immune response, affects cancer immune response. Passlick and Izbicki et al.—who first reported comparative clinical randomized trial between SLND and LNS—also analyzed postoperative immune responses (27). In an analysis of 50 resected NSCLC cases, the expression of major histocompatibility complex (MHC) class I and intracellular adhesion molecule (ICAM)-1 in cancer cells decreased in cases with cancer progression in lymphoid tissues.
We also established cancer cell lines from the primary tumor and subcutaneous metastasis in a resected case of esophageal cancer, and reported that ICAM-1 expression decreased in the subcutaneous metastasis to avoid cytotoxic T lymphocyte (CTL) attack (28). In addition, lung cancer cell lines in resected NSCLC patients and induced cancer-specific CTL clones from autologous lymph node lymphocytes obtained by lymph node dissection were established. We analyzed the mechanism by which established CTL clones kill lung cancer cell lines, and identified six cancer antigens recognized by cancer-specific CTL clones (29-34). By reproducing the reaction occurring in vivo in NSCLC patients in vitro, we confirmed that cancer-specific CTLs exist in the regional lymph nodes and kill the autologous lung cancer cells. We have demonstrated that CTL that recognizes p53 mutation and attack lung cancer cells cannot be detected in peripheral blood, but is present in regional lymph node and tumor, and induces anti-tumor immunity in a lung cancer case (29).
At present, as immune checkpoint inhibitors (ICIs) are showing excellent effects (35-38), it may be necessary to examine the impact of removing regional lymph nodes where cancer-specific CTLs are concentrated. ICIs activate immune cells present in regional lymph nodes and exerts its effects. Of course, SLND is essential for accurate staging at this time, but ideally, in a host with no cancer cells in the lymph node or a host with cancer cells having a high sensitivity to ICI, it might be accepted to leave the regional lymph node in the future from our basic research (29-34).
Notably, it was recently clarified that the addition of ICI to postoperative adjuvant chemotherapy for NSCLC significantly prolongs DFS (39), and this was applied clinically. Still further, the need for an immunological reconsideration of regional lymph nodes increased.
Future lymph node dissection for lung cancer surgery
Pathological N0 lung cancer has increased in recent years due to advances in imaging technology. It is also thought that the number of cases in which lymph node dissection is practically unnecessary is increasing. It seems necessary to consider the individualization and miniaturization of lymph node dissection. It will also be necessary to examine the significance of lymph node dissection in limited surgery such as segmentectomy for peripheral small-sized lung cancer.
On the other hand, the role of regional lymph nodes in lung cancer immunity also needs to be elucidated. It is also necessary to verify whether leaving lymph nodes without metastasis can suppress the occurrence of secondary lung cancers and pneumonia, and whether the presence of regional lymph nodes can bring out the effects of ICI treatment.
Conclusions
The diagnostic significance of regional lymph nodes is clear, but the prognostic significance still remains controversial. Although SLND has been the international standard, it may not be the best option in all cases. The time may come when the extent of lymph node dissection is determined individually for each case. Future verification results are awaited.
Acknowledgments
Funding: Ichiki acknowledges grant support from JSPS KAKENHI (Nos. 18K08806, 19K09294, and 22K09013).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1527/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1527/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1527/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Goldstraw P. Report on the international workshop on intrathoracic staging, London, October, 1996. Lung Cancer 1997;18:107-11. [Crossref]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Naruke T, Tsuchiya R, Kondo H, et al. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg 1999;16:S17-24. [Crossref] [PubMed]
- Cahan WG, Watson WL, Pool JL. Radical pneumonectomy. J Thorac Surg 1951;22:449-73. [Crossref] [PubMed]
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Sakaguchi H, Ikeda S, Kawano R, et al. Surgical treatment of N2 involved non-small cell lung cancer--the systematic extended lymph node dissection based on the regional lymphatic drainage. Kyobu Geka 1999;52:901-5. [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [Crossref] [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Wu YL, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Zhang J, Mao T, Gu Z, et al. Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: Results of a prospective randomized trial. Thorac Cancer 2013;4:416-21. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Nohl HC. An investigation into the lymphatic and vascular spread of carcinoma of the bronchus. Thorax 1956;11:172-85. [Crossref] [PubMed]
- Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Maniwa T, Okumura T, Isaka M, et al. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e59-64. [Crossref] [PubMed]
- Hishida T, Saji H, Watanabe SI, et al. A randomized Phase III trial of lobe-specific vs. systematic nodal dissection for clinical Stage I-II non-small cell lung cancer (JCOG1413). Jpn J Clin Oncol 2018;48:190-4. [Crossref] [PubMed]
- Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Schlachtenberger G, Doerr F, Menghesha H, et al. Sublobar resection without staging and lymphadenectomy for ≤2 cm Non-Small Cell Lung Cancer is no adequate therapy. Surg Oncol 2022;44:101840. [Crossref] [PubMed]
- Passlick B, Pantel K, Kubuschok B, et al. Expression of MHC molecules and ICAM-1 on non-small cell lung carcinomas: association with early lymphatic spread of tumour cells. Eur J Cancer 1996;32A:141-5. [Crossref] [PubMed]
- Ichiki Y, Hanagiri T, Takenoyama M, et al. Differences in sensitivity to tumor-specific CTLs between primary and metastatic esophageal cancer cell lines derived from the same patient. Surg Today 2012;42:272-9. [Crossref] [PubMed]
- Ichiki Y, Takenoyama M, Mizukami M, et al. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol 2004;172:4844-50. [Crossref] [PubMed]
- So T, Takenoyama M, Mizukami M, et al. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res 2005;65:5945-52. [Crossref] [PubMed]
- Nagata Y, Hanagiri T, Takenoyama M, et al. Identification of the HLA-Cw*0702-restricted tumor-associated antigen recognized by a CTL clone from a lung cancer patient. Clin Cancer Res 2005;11:5265-72. [Crossref] [PubMed]
- Fukuyama T, Hanagiri T, Takenoyama M, et al. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res 2006;66:4922-8. [Crossref] [PubMed]
- Takenoyama M, Baurain JF, Yasuda M, et al. A point mutation in the NFYC gene generates an antigenic peptide recognized by autologous cytolytic T lymphocytes on a human squamous cell lung carcinoma. Int J Cancer 2006;118:1992-7. [Crossref] [PubMed]
- Sugaya M, Takenoyama M, Shigematsu Y, et al. Identification of HLA-A24 restricted shared antigen recognized by autologous cytotoxic T lymphocytes from a patient with large cell carcinoma of the lung. Int J Cancer 2007;120:1055-62. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]


