
Immune checkpoint inhibitors beyond first-line progression with prior immunotherapy in patients with advanced non-small cell lung cancer
Highlight box
Key findings
• While the benefits of continuing prior immune checkpoint inhibitor administration beyond first-line immunotherapy progression might be limited for patients with advanced NSCLC, this treatment regime could be considered in patients who achieve better efficacy before first-line PD.
What is known and what is new?
• The benefits of continuing prior ICI administration beyond first-line immunotherapy progression have always been controversial.
• This is one of the earliest studies conducted on individuals who continued the original ICIs after initial PD.
What is the implication, and what should change now?
• Continuing the same ICIs may be an alternative for patients who achieve better efficacy before first-line PD.
Introduction
Lung cancer is one of the most commonly diagnosed malignancies and a main cancer-related mortality worldwide (1), of which non-small cell lung cancer (NSCLC) makes up 80–85% (2). An increase in the incidence and mortality of NSCLC in China has been observed in recent years (3). Indeed, NSCLC is emerging as a global health challenge (4).
With immune escape mechanisms under investigation, gold treatment guidelines for advanced driver gene-negative NSCLC patients in China incorporated immune checkpoint inhibitor (ICI) monotherapy, including programmed cell death protein 1/programmed cell death-ligand 1 inhibitor monotherapy, and ICIs plus platinum-based doublet chemotherapy (5). In most clinical trials (6-9), such as keynote 024, checkmate 227, and so on, the survival rate of advanced NSCLC improves dramatically as a result of immunotherapy. However, continuing with ICIs with follow-up treatment regimens is still under investigation for advanced NSCLC patients on whom immunotherapy has failed.
Although immunotherapy beyond progression (IBP) among advanced NSCLC patients progressed from prior immunotherapy has been under investigation, majority of the studies included only small sample sizes. In some retrospective analyses, immunotherapy beyond initial progression has been associated with longer overall survival (OS) and progression-free survival (PFS) in patients with advanced NSCLC, which may indicate a novel therapeutic schedule. Some clinical trials have shown the opposite, however, with no statistical difference associated with continued immunotherapy. Much remains to be understood, particularly in terms of treatment beyond first-line immunotherapy.
Collectively, a retrospective research under real-world circumstances was carried out to assess the efficacity of continuing the same ICIs in advanced NSCLC patients after progression of first-line immunotherapy. The results confirm it is beneficial to certain patients in terms of clinical features. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1611/rc).
Methods
Study design and patients
Medical records of 267 patients were reviewed; they were all with pathologically or cytologically advanced NSCLC (IIIB to IV) and recurrent NSCLC evaluated as progression disease (PD) after receiving first-line immunotherapy combined with platinum-based chemotherapy at Zhejiang Cancer Hospital (Hangzhou, China) between November 2017 and July 2021. All the patients with lung cancer were staged in accordance with the 8th TNM classification. Retrospective demographics, clinical, and radiological information were extracted from electronic medical records (EMRs).
Inclusion criteria were as follows: measurable lesions defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; Eastern Cooperative Oncology Group (ECOG) performance status 0–1; and adequate organ and bone marrow reserved. Exclusion criteria were incomplete EMRs, prior malignancies, and enrollment in clinical trials.
Patients who were administered with the same immunotherapy scheme in second-line treatment post first PD were defined as IBF, while those who discontinued the immunotherapy for other treatments such as chemotherapy, radiotherapy, or chemoradiotherapy were defined as non-IBF. Furthermore, based on the relationship between PFS1 and 6 months, patients were separated into group A (PFS1 ≤6 months) and group B (PFS1 >6 months).
The study protocol was approved by the Ethics Committee at Zhejiang Cancer Hospital (Approval No. IRB-2022-187) and was carried out in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Treatment and response assessments
The clinical responses, including complete response (CR), partial response (PR), stable disease (SD), and PD, were evaluated using computed tomography (CT) based on RECIST v1.1. PFS1 was defined as the time from the initiation of first-line immunotherapy to the date of confirmed PD. PFS2 was defined as the period from the first day of second-line immunotherapy to progression or all-cause mortality, whichever occurred first. OS was defined as the time from the first PD until death, loss to follow-up, or final follow-up. The objective response rate (ORR) was defined as the sum of the CR and PR, while the disease control rate (DCR) was the sum of the CR, PR, and SD. The date of the last follow-up was September 3, 2022.
Statistical analysis
Percentages (%) was presented to depict the baseline demographic statistics. Pearson’s Chi-squared or Fisher’s exact test was used to compare categorical variables in baseline characteristics between groups. Student’s t-test or Mann-Whitney U test was adopted to evaluate the differences in continuous or ordinal variables. Kaplan-Meier curves were applied to calculate median PFS1, PFS2, and OS, and the log-rank test was used to evaluate differences. The multivariable Cox proportional hazard regression model was applied to determine the hazard ratio (HR) and corresponding 95% confidence interval (CI), and case characteristics with significant outcomes were regarded as independent predictive factors. P values were calculated given a two-sided hypothesis, and P<0.05 was considered statistically significant. Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Patients
This retrospective study finally screened out 94 patients with advanced NSCLC who received PD post first-line treatment with platinum-based doublet chemotherapy plus immunotherapy between November 2017 and July 2021. The specific screening flow chart is shown in Figure 1. Baseline characteristics of patients are summarized in Table 1. The median age was 63 years (range, 33–76 years) old, with 33 (35.1%) patients aged above 65; 71 (75.5%) patients were male, while 23 (24.5%) were female. Thirty-one (33.0%) patients were never-smokers. The histologic subtypes present were adenocarcinoma in 45 (47.9%) patients and non-adenocarcinoma in 47 (52.1%) patients. Liver and brain metastases at diagnosis were 19 (20.2%) and 12 (12.8%), respectively. The ECOG performance status of each patient was 0 or 1. Subjects were divided into two groups as per whether the same immunotherapy was continued following PD of first-line therapy: IBF (n=42) and non-IBF (n=52). Detailed characteristics of two groups were listed in Table 1.
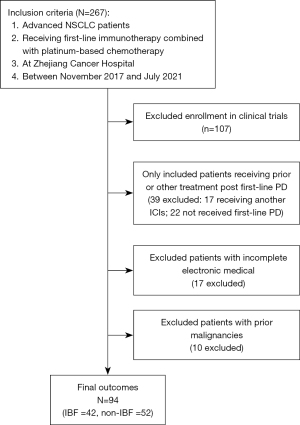
Table 1
| Characteristics | No. of patients (%) | ||
|---|---|---|---|
| All patients (n=94) | Non-IBF (n=52) | IBF (n=42) | |
| Age (years) | |||
| ≤65 | 61 (64.9) | 34 (65.4) | 27 (64.3) |
| >65 | 33 (35.1) | 18 (34.6) | 15 (35.7) |
| Sex | |||
| Male | 71 (75.5) | 37 (71.2) | 34 (81.0) |
| Female | 23 (24.5) | 15 (28.8) | 8 (19.0) |
| ECOG performance status | |||
| 0 | 23 (24.5) | 15 (28.8) | 8 (19.0) |
| 1 | 71 (75.5) | 37 (71.2) | 34 (81.0) |
| Smoking history | |||
| Ever | 63 (67.0) | 33 (63.5) | 30 (71.4) |
| Never | 31 (33.0) | 19 (36.5) | 12 (28.6) |
| Histology | |||
| Squamous | 36 (38.3) | 19 (36.5) | 17 (40.5) |
| Adenocarcinoma | 45 (47.9) | 30 (57.8) | 15 (35.7) |
| Adenosquamous | 4 (4.3) | 1 (1.9) | 3 (7.1) |
| Unknown | 9 (9.5) | 2 (3.8) | 7 (16.7) |
| PD-L1 expression | |||
| Negative (<1%TPS) | 12 (12.8) | 5 (9.6) | 7 (16.7) |
| Low (1–49%TPS) | 22 (23.4) | 11 (21.2) | 11 (26.2) |
| High (≥50%TPS) | 11 (11.7) | 4 (7.7) | 7 (16.7) |
| Not tested | 49 (52.1) | 32 (61.5) | 17 (40.4) |
| Liver metastases | |||
| Yes | 19 (20.2) | 8 (15.4) | 11 (26.2) |
| No | 75 (79.8) | 44 (84.6) | 31 (73.8) |
| Brain metastases | |||
| Yes | 12 (12.8) | 10 (19.2) | 2 (4.8) |
| No | 82 (87.2) | 42 (80.8) | 40 (95.2) |
| Bone metastases | |||
| Yes | 45 (47.9) | 23 (44.2) | 22 (52.4) |
| No | 49 (52.1) | 29 (55.8) | 20 (47.6) |
IBF, immunotherapy beyond first-line progression; non-IBF, non-immunotherapy beyond first-line progression; ECOG performance status, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death-ligand 1; TPS, tumor cell proportion score.
Treatment response
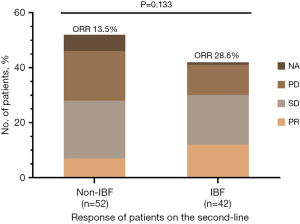
All the patients involved in the study exhibited RECIST v1.1 PD to first-line immunotherapy consistent with the inclusion criteria. A summary of the confirmed best overall response before and post-first progression is listed in Table 2 and Figure 2. In the first-line treatments, 18 (34.6%) patients achieved PR and 26 (50.0%) had SD in the IBF subgroup, compared to 17 (40.5%) and 19 (45.2%), respectively, in the non-IBF group. A similar result was observed in second-line treatments; 7 (13.5%) PR and 21 (40.4%) SD patients were in the former compared to 12 (28.6%) PR and 18 (42.8%) in the latter. No difference was found before or post-first progression between the IBF and non-IBF groups in terms of ORR (first-line treatment: 34.6% vs. 40.5%, P=0.559; second-line treatment: 13.5% vs. 28.6%, P=0.070, respectively).
Table 2
| Type of response | No. of patients (%) | P value | ||
|---|---|---|---|---|
| All patients (n=94) | Non-IBF (n=52) | IBF (n=42) | ||
| The first-line | 0.842 | |||
| Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Partial response | 35 (37.2) | 18 (34.6) | 17 (40.5) | |
| Stable disease | 45 (47.9) | 26 (50.0) | 19 (45.2) | |
| Progressive disease | 14 (14.9) | 8 (15.4) | 6 (14.3) | |
| Objective response ratea (95% CI) | 35 (37.2) | 18 (34.6) | 17 (40.5) | 0.559 |
| Disease control rateb (95% CI) | 80 (85.1) | 44 (84.6) | 36 (85.7) | 0.882 |
| The second-line | 0.121 | |||
| Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Partial response | 19 (20.2) | 7 (13.5) | 12 (28.6) | |
| Stable disease | 39 (41.5) | 21 (40.4) | 18 (42.8) | |
| Progressive disease | 29 (30.9) | 18 (34.6) | 11 (26.2) | |
| Not evaluated | 7 (7.4) | 6 (11.5) | 1 (2.4) | |
| Objective response ratea (95% CI) | 19 (20.2) | 7 (13.5) | 12 (28.6) | 0.070 |
| Disease control rateb (95% CI) | 58 (61.7) | 28 (53.9) | 30 (71.5) | 0.081 |
a, complete response or partial response; b, complete response, partial response, or stable disease. IBF, immunotherapy beyond first-line progression; non-IBF, non-immunotherapy beyond first-line progression; 95% CI, 95% confidence interval.
Efficacity analyses
A total of 15 (16.0%) individuals were lost to follow-up, including 4 (9.5%) patients in the IBF group, which did not significantly affect the study.
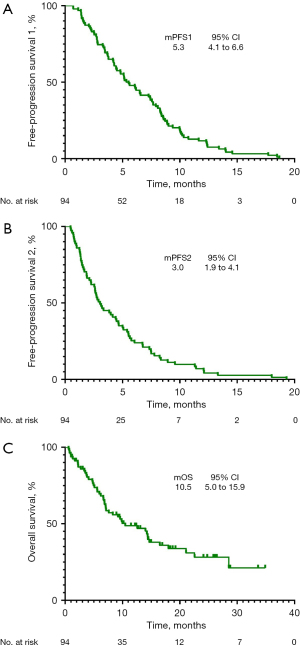
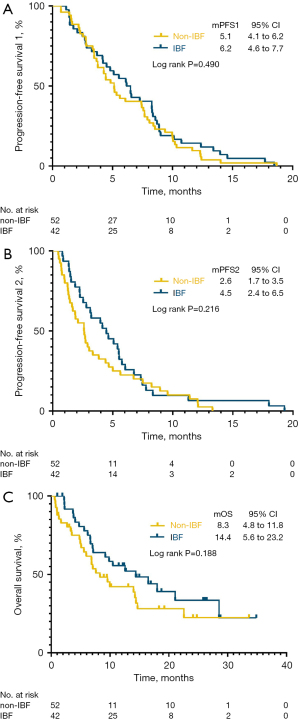
The overall population median PFS1 was 5.3 months (95% CI: 4.1 to 6.6 months), median PFS2 was 3.0 months (95% CI: 1.9 to 4.1 months), and median OS was 10.5 months (95% CI: 5.0 to 15.9 months) (Figure 3). PFS1 was statistically similar among the IBF and non-IBF groups (PFS1: 6.2 vs. 5.1 months, P=0.490) (Figure 4A), indicating the balance of primal survival. However, no significant difference was observed in PFS2 (4.5 vs. 2.6 months, P=0.216) (Figure 4B) and OS (14.4 vs. 8.3 months, P=0.188) (Figure 4C), demonstrating that continuation of immunotherapy may have no effect on survival.
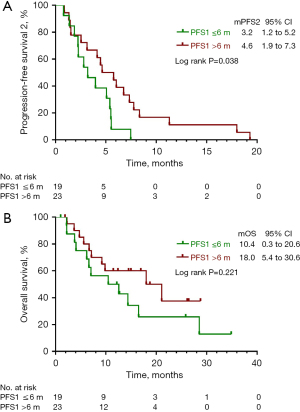
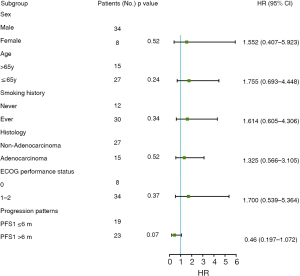
Further analysis was carried on patients who achieved PFS1 ≤6 months (group A) and PFS1 >6 months (group B) during first-line therapy. Compared with group B, patients who achieved a longer PFS1 may present with a better curative outcome, with a median PFS2 of 3.2 months (95% CI: 1.2 to 5.2 months) in contrast to 4.6 months (95% CI: 1.9 to 7.3 months) in PFS1 ≤6 months patients (P=0.038) (Figure 5A). Although there was no statistically significant difference in OS between the two groups (P=0.221), the subgroup analysis showed that patients in group A (mOS: 10.4, 95% CI: 0.3 to 20.6 months) exhibited a favorable trend in OS compared with group B (mOS: 18.0, 95% CI: 5.4 to 30.6 months) (Figure 5B). Furthermore, multivariate analysis revealed that variables including sex, age, smoking history, histology, ECOG performance status, and progression patterns failed to independently impact second-line free-progression survival (Figure 6).
Discussion
The present study provided analysis of treatment choices in subsequent therapy after first-line failure for advanced NSCLC patients, and showed limited benefits of continuing prior ICI administration beyond first-line immunotherapy progression, except for cases with a longer maintenance period during the first-line therapy.
With immunotherapy bringing a paradigm shift in oncology treatment, treatment regimens for diverse conditions are also under development. Discoveries from initial monotherapy, combination therapy, and subsequent IBP are being kept abreast of scientific development. Some treatment patterns have shown noteworthy survival benefits, such as the PACIFIC model (10).
A similar result has been observed in some IBP studies. A retrospective study from Ge et al. (11) reported IBP may enable patients with advanced NSCLC to achieve prolonged OS (median: 26.6 vs. 10.7 months; P=0.015) and PFS (median: 9.7 vs. 4.3 months; P<0.001). Regardless of the fact that there was no statistically significant difference in the ORR (15.4% vs. 11.6%, P=0.560), the DCR was considerably higher in the IBP group (89.7% vs. 61.6%, P=0.001). Similarly, according to another retrospective analysis with 60 patients, Ricciuti et al. (12) revealed that for a selected group of patients with advanced NSCLC, continuing nivolumab may provide promising clinical survival benefits, with a median OS of 17.8 months.
Nevertheless, ICI retreatment after progression remains controversial as some studies have shown opposite results. David et al. (13) conducted a retrospective analysis in which the median post-PD OS was 12.7 months in 168 atezolizumab-administered patients in IBP, while 8.8 months in 94 patients switching to non-protocol therapy. Enomoto et al. (14) showed no remarkable advantages relevant to continuation of nivolumab for advanced NSCLC patients (15.6 vs. 13.4 months, P=0.40). A summary of relevant studies is listed in Table 3. In this context, the curative effects of IBP, especially continuing the same ICI post first-line PD, remain to be determined.
Table 3
| Reference | Regimen | IBP | Non-IBP | P value | |||
|---|---|---|---|---|---|---|---|
| N | OS | N | OS | ||||
| Ge et al. (11) | PD-(L)1 | 39 | 26.6 | 86 | 10.7 | 0.015 | |
| Ricciuti et al. (12) | Nivolumab | 60 | 17.8 | 116 | 3.7 | <0.001 | |
| Enomoto et al. (14) | Nivolumab | 28 | 15.6 | 46 | 13.4 | 0.40 | |
| David et al. (13) | Atezolizumab | 168 | 12.7 | 94 | 8.8 | NE | |
IBF, immunotherapy beyond first-line progression; non-IBF, non-immunotherapy beyond first-line progression; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; OS, overall survival; NE, not evaluated.
Hence, this retrospective study has been designed. The study, to our knowledge, is one of the earliest studies conducted on individuals who were given an immunotherapy regime as first-line treatment, and evaluates the effect of continuing the same ICIs beyond first progression. Little survival benefits were revealed in continuing ICI administration beyond first-line immunotherapy, as shown by the statistical outcomes between PFS1, PFS2, and OS. These analyses lead us to further explore this new therapeutic strategy. To investigate relevant clinical characteristics, this retrospective study focused on the association between first- and second-line survival for patients with advanced NSCLC. Subgroup analyses indicated that prolonged duration of first-line therapy provided a longer PFS2 and a favorable tendency for OS. The promising consequence of PFS2 may encourage us to consider this regime for those with better survival in the first-line immunotherapy. OS is influenced by many factors, which requires more prospective studies. A multivariate analysis, which showed no statistical differences, was also conducted, indicating longer first-line duration cannot predict or independently influence survival.
The present study also has some limitations. First, it is limited by its retrospective and single-center design, as well as the small sample size, which may undermine the reliability of the results by recall and selection bias. Second, the clinical response, subsequent treatment regime, and types of ICIs were only evaluated by clinicians, so we cannot adequately estimate the real impact of IBF. Third, the majority of patients lacked tumor mutation burden or PD-L1 expression, which may impact further research on predictive biomarkers. Lastly, this study did not use immune-related RECIST (irRECIST), although it has been suggested to be too complicated to be widely used in clinical practice (14). Thus, there is still much room for IBP and more prospective studies are required.
Conclusions
There were no significant benefits associated with continuation of original ICIs for advanced NSCLC patients beyond first-line immunotherapy. However, this treatment regime might be considered for patients who show better outcomes before first-line PD. Large prospective clinical trials are required to further validate these findings.
Acknowledgments
The authors would like to acknowledge all patients and their families for their cooperation and participation. Additionally, we are thankful to all research staff and co-investigators involved in this study. We also thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: The study was supported by the Medical Scientific Research Foundation of Zhejiang Province (No. 2022KY653) and sponsored by the Zhejiang provincial program for the Cultivation of High-Level Innovative Health Talents (Zhengbo Song).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1611/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1611/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1611/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee at Zhejiang Cancer Hospital (Approval No. IRB-2022-187) and was carried out in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol 2020;21:e342-9. [Crossref] [PubMed]
- Zhou F, Zhou CC. Immunotherapy in non-small cell lung cancer: advancements and challenges. Chin Med J (Engl) 2021;134:1135-7. [Crossref] [PubMed]
- Chai Y, Wu X, Zou Y, et al. Immunotherapy combined with chemotherapy versus chemotherapy alone as the first-line treatment of PD-L1-negative and driver-gene-negative advanced nonsquamous non-small-cell lung cancer: An updated systematic review and meta-analysis. Thorac Cancer 2022;13:3124-32. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol 2022;17:289-308. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Ge X, Zhang Z, Zhang S, et al. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res 2020;9:2391-400. [Crossref] [PubMed]
- Ricciuti B, Genova C, Bassanelli M, et al. Safety and Efficacy of Nivolumab in Patients With Advanced Non-small-cell Lung Cancer Treated Beyond Progression. Clin Lung Cancer 2019;20:178-185.e2. [Crossref] [PubMed]
- Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab Treatment Beyond Progression in Advanced NSCLC: Results From the Randomized, Phase III OAK Study. J Thorac Oncol 2018;13:1906-18. [Crossref] [PubMed]
- Enomoto T, Tamiya A, Matsumoto K, et al. Nivolumab treatment beyond progressive disease in advanced non-small cell lung cancer. Clin Transl Oncol 2021;23:582-90. [Crossref] [PubMed]


