
The effect of chest wall surgery on lung volume: a new evaluation concept
Highlight box
Key findings
• Lung volume measured by computed tomography can be useful in evaluating the effectiveness of chest wall surgery.
What is known and what is new?
• In the surgical treatment of chest wall tumors that require major resection of the chest wall, chest wall defects are reconstructed using a variety of materials and techniques; however, there is currently no adequate method to assess whether each reconstruction is successful.
• Comparison of lung volume measurements by computed tomography before and after surgery might be useful in assessing thoracic cage morphology and the maintenance of lung expansion.
What is the implication, and what should change now?
• Measuring the rate of change in lung volume before and after surgery is useful for evaluating the effectiveness of chest wall surgery.
• In the future, this may contribute to the generalization of the currently diverse selection of reconstructive materials and techniques.
Introduction
The integrity and stability of the chest wall (CW) are major factors in ensuring the protection of the thoracic organs and proper respiratory function. Thoracic surgeons often deal with neoplastic, traumatic, and malformative diseases that affect the CW and require destruction, reconstruction, or stabilization of the thorax (1). CW reconstruction has developed significantly with advances in surgical techniques and the availability of various prostheses and biomaterials (2,3). However, each prosthetic material has advantages and disadvantages, and none has been proven superior. Furthermore, no clear guidelines for managing CW disease exist yet. One reason for this is the lack of reliable postoperative evaluation methods.
The success of CW reconstruction has been considered in terms of respiratory function, thoracic deformity, and rigidity (4-6). However, to date, there has been no report of an appropriate method to evaluate the negative effect on lung expansion, which is directly attributed to thoracic deformity and rigidity and also affects respiratory function. Therefore, we defined the effectiveness of chest wall surgery as maintaining sufficient rigidity of the chest wall and lung expansion and sought a new imaging evaluation method by measuring the lung volume (LV) before and after the resection of CW tumors. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1580/rc).
Methods
Study participants
We conducted a retrospective chart review of patients with CW tumors who underwent curative surgery at Chiba University Hospital between 2004 and 2017. Patients with lung excision, no computed tomography (CT) image after surgery, a second surgery, or a history of pneumonectomy for lung cancer were excluded. All the patients underwent regular follow-ups at our outpatient clinic after discharge. All patients’ medical histories, surgical records, and radiological and pathological results were reviewed. The pathological diagnosis was determined according to the World Health Organization histological typing (7). The reconstruction method was divided into rigid reconstruction [a combination of titanium mesh and extended polytetrafluoroethylene (ePTFE) sheet], non-rigid reconstruction (ePTFE sheet only), non-reconstruction with CW defect, and no resected CW cases (tumor extirpation only). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee at Chiba University (No. 2365). Because this was a retrospective study, we used an opt-out method and included participants unless they explicitly expressed their willingness to be excluded, in accordance with the Japanese ethical guidelines (8). The requirement for individual patient consent for data collection was waived by the Institutional Review Board.
Measurement of the change rate in LV
LV before and after surgery was measured using three-dimensional (3D)-CT. 3D-CT analysis and lung volumetry were performed using SYNAPSE VINSENT (FUJIFILM, Tokyo, Japan) (Figure S1A). In principle, postoperative CT images more than six months after the operation were used for analysis. LV and preoperative and postoperative LV changes were compared for each reconstruction method.
The rate of change in LV was calculated as the corrected postoperative LV of the operative side/ preoperative LV of the operative side. The corrected postoperative LV of the operative side was calculated as the postoperative LV of the operative side × preoperative/postoperative LV of the opposite side. The timing and conditions of breathing when CT was performed were corrected using the LV of the opposite side.
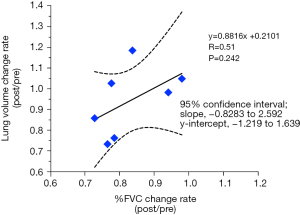
The correlation between the rate of change (%) in LV and the rate of change (%) of forced vital capacity was also examined in patients who underwent preoperative and postoperative respiratory testing.
Calculation of excised chest wall area
The excised CW area was calculated as vertical diameter × horizontal diameter of the tissue specimen (Figure S1B). In cases where CW excision was not performed, the excised CW area was calculated as zero. The mean excised CW area was compared for each reconstructive method.
Statistical analysis
Data are presented as the mean ± standard deviation. Student’s t-test was used for continuous variables. Preoperative and postoperative LV were compared using a paired t-test. Correlation analysis was performed to identify the relationship between the rate of change in the LV and the excised CW area. All tests were two-sided. Statistical significance was set at a P value <0.05; however, in the case of comparing each pair, statistical significance was set at P value <0.01. Statistical analyses were performed using JMP (SAS, Cary, NC, USA) and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
Results
Forty patients with CW tumors who underwent curative surgery during the study period were included. Of these 40 patients, seven with lung resection, six without postoperative CT images, three who underwent a second surgery, and one with a history of pneumonectomy due to lung cancer were excluded. Finally, 23 patients were included in this study (Figure 1).
The clinicopathological characteristics of the patients are summarized in Table 1. Primary CW tumors occurred in 15 patients (65%) and metastatic CW tumors in eight patients (35%). Of the 15 patients with primary CW tumors, 9 (60%) had benign tumors, and 6 (40%) had malignant tumors. The reconstruction methods were rigid reconstruction (a combination of titanium mesh and ePTFE sheet) in four patients, non-rigid reconstruction (ePTFE sheet only) in 11, non-reconstruction in five, and no CW resection in three (tumor extirpation only). No cases of sternal reconstruction were identified.
Table 1
| Variable | Category | Patients with CW tumor (n=23) | Subcategory | Number of subcategories | Remarks |
|---|---|---|---|---|---|
| Age, mean ± SD | 60.9±15.3 | ||||
| Gender | Female | 7 (30%) | |||
| Side | Left | 8 (35%) | |||
| Pathological diagnosis | Primary | 15 (65%) | Benign | 9 (39%) | Schwannoma n=3, desmoid n=2, lipoma n=2, cavernous hemangioma n=1, SFT n=1 |
| Malignant | 6 (26%) | Chondrosarcoma n=3, liposarcoma n=1, myxofibrosarcoma n=1, lymphoma n=1 |
|||
| Metastasis | 8 (35%) | HCC n=3, lung cancer n=2, MPNST n=1, skin cancer n=1, primary unknown n=1 |
|||
| Reconstruction method | Rigid | 4 (17%) | Titanium mesh & ePTFE sheet | ||
| Non-rigid | 11 (48%) | ePTFE sheet | |||
| No reconstruction | 5 (22%) | ||||
| No chest wall resection | 3 (13%) |
Continuous data are presented as mean ± standard deviation and categorical data as numerical values (percentage of total 23 patients). SD, standard deviation; CW, chest wall; SFT, solitary fibrous tumor; HCC, hepatocellular carcinoma; MPNST, malignant peripheral nerve sheath tumor; ePTFE, expanded polytetrafluoroethylene.
The CW resection area for each reconstruction procedure is shown in Figure 2 and summarized in Table 1. Rigid reconstruction was applied in cases with larger resections >150 cm2. Non-rigid reconstruction was applied in cases with resection areas of 30–90 cm2, and non-reconstruction was applied in cases with resection areas of 40 cm2 or less.
The rate of change in the LV for each reconstruction procedure is shown in Figure 3 and Table 2. The median time from preoperative CT to surgery was 12 days [interquartile range (IQR), 1–3: 4.5–44.5], and the median time from surgery to postoperative CT was 347 days (IQR, 1–3: 181–437). The mean LV in the no-CW excision cases decreased significantly after surgery. The mean LV in the rigid reconstruction cases decreased from 2,159 to 1,791 mL; however, this change was insignificant. The non-rigid and non-reconstruction cases did not change before and after surgery (Figure 3A). However, the rate of change in the LV in the rigid reconstruction cases was significantly lower than that in the non-rigid reconstruction cases (Figure 3B).
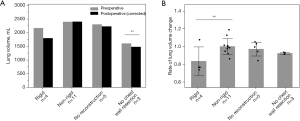
Table 2
| Variable | All (n=23) | Rigid (n=4) | Non-rigid (n=11) | No reconstruction (n=5) | No CW resection (n=3) |
|---|---|---|---|---|---|
| Resected CW area (cm2) | 44.2 (0–188.8) | 172.8 (55.3–188.8) | 61.8 (33.8–82.5) | 26.2 (12.7–32.5) | 0 (0–0) |
| Operative side preoperative LV (cm3) | 2,225±510 | 2,159±548 | 2,388±457 | 2,293±481 | 1,600±21 |
| Operative side postoperative LV (cm3) | 2,079±547 | 1,676±486 | 2298 ± 478 | 2,237±541 | 1,548±298 |
| Opposite-side preoperative LV (cm3) | 2,249±539 | 2,311±356 | 2,386±336 | 2,335±414 | 1,518±288 |
| Opposite-side postoperative LV (cm3) | 2,196±534 | 2,182±445 | 2284±297 | 2,367±399 | 1,612±496 |
| LV change (%) | 95.4±11.1 | 83.3±16.1 | 99.9±9.0 | 96.9±12.7 | 92.2±1.1 |
Values are presented as mean ± standard deviation or median (range). CW, chest wall; LV, lung volume; SD, standard deviation.
Correlation analysis was performed to identify the relationship between the rate of change in the LV and the excised CW area. A weak correlation was observed between the excised CW area and the rate of change in the LV (|r| =0.26). The approximate straight line of the CW excision area and the rate of change in the LV was y =0.98453-0.00053x; however, the correlation was insignificant (P=0.23) (Figure 4). Changes in the LV were generally well preserved, regardless of the excised CW area. There was a large decrease in LV in some non-rigid and rigid reconstruction cases. In addition, there were cases where the reconstructive material migrated into the thorax due to postoperative lung inflammation and contraction and where the reconstructive material was deflected.
Seven cases in which respiratory function was measured before and after surgery were observed. A positive but insignificant correlation was observed between the percent change in lung capacity and the percent change in forced lung capacity (Figure 5).
The excised CW area tended to be larger in the anterior sites than in the posterior site, but the rates of change in LV were almost equivalent among the resection sites (Figure S2A,S2B). In addition, there was no significant correlation between the excised CW area or the rates of change in LV and body mass index (Figure S3A,S3B).
Discussion
Ideally, CW reconstruction is considered to preserve the thoracic cage and respiratory function postoperatively. Therefore, synthetic resins, biomaterials, and metal materials have been used to reconstruct chest wall defects (2,9-16). However, each prosthetic material has its advantages and disadvantages, and none has been proven superior (17). Furthermore, their superiority remains unclear because no studies have been conducted to compare their results. Therefore, CW reconstruction has been performed in various ways in many institutions. In this study, we investigated whether the superiority or inferiority of reconstruction methods could be examined by measuring LV before and after surgery.
According to our results, the postoperative LV was maintained in cases of non-rigid reconstruction and non–reconstruction. In contrast, the postoperative LV in rigid reconstruction cases was lower than that in non-rigid reconstruction cases in Figure 3B. The rate of change in LV was 92.2%±1.1%, even in patients without CW resection, suggesting that postoperative inflammation and adhesion could cause a constant decrease in LV.
If the ideal reconstruction method completely reconstructs the thorax, the tumor that has extended into the thorax and is compressing the lungs will be removed, resulting in restoration of lung expansion. Thus, postoperative LV should theoretically be equal to or greater than preoperative LV. This tendency becomes clearer as the tumor volume increases, because a large tumor compresses the normal lung. However, it was not desirable for us that the LV be reduced using rigid reconstruction in this study, and there seems to be room for improvement in our reconstruction techniques from that point of view. Furthermore, upon re-examination of postoperative CT images, we observed decreased lung expansion with migration and deflection of the reconstructive material into the thorax due to postoperative lung inflammation and shrinking. These cases could allow us to reconsider new reconstructive techniques that retain more rigidity and allow normal physiological movement of the chest wall, such as an artificial rib system (18-20). It was shown that the presence and degree of this divergence could lead to an evaluation of the procedure for CW reconstruction.
The CW reconstruction method was significantly associated with the CW resection area. Previous reports have shown that reconstruction is often required when four or more ribs are resected and a defect greater than 5 cm in diameter is observed or when thoracic instability is suspected, even if the defect is small (5,21-23). Furthermore, anterior and anterolateral movements are greater than posterior ones, often requiring thoracic reconstruction (24).
At our hospital, the indication for CW reconstruction is decided at a conference based on a comprehensive evaluation of the resection site and the number of ribs removed. The results of this study confirmed that non-rigid reconstruction could be applied to small defects and rigid reconstruction to large defects.
The advantage of this analysis method is that a universal representation is objectively provided by the number. Furthermore, because CT before and after surgery is often performed in any facility, it is possible to compare the superiority or inferiority of the reconstruction procedure beyond the facility. In the future, further investigation is required to provide robust evidence on the techniques for CW reconstruction using the present image examination in many facilities.
Relevant limitations of this monocentric study are the retrospective design of the data and the relatively small number of patients. In addition, the usefulness of this image evaluation method is unclear because no test method has been established as the gold standard. Moreover, this study was unable to evaluate preoperative respiratory function. In this study, the postoperative follow-up method was not standardized. This is because respiratory function was measured after surgery in only seven of the 23 cases. If data on the forced vital capacity before and after surgery are available, the effect of CW surgery on respiratory function can be well investigated. Our preliminary data on the percentage of forced volume capacity suggests that this pulmonary volumetry analysis could be a surrogate parameter. Furthermore, several reports have studied respiratory function after CW excision; however, because the number of cases is small, it is impossible to conclude the effect of CW surgery on respiratory function (16,25-27). It can also be argued that the results of preoperative and postoperative LV changes may be influenced by various factors, including the postoperative course, surgical factors, postoperative therapy, and changes in the respiratory phase, and are not simply a direct result of changes due to reconstructive surgery. The total was divided by the contralateral LV change rate to adjust for changes in the respiratory phase; however, the accuracy of these formulas needs to be further studied.
Conclusions
In this study, we demonstrated lung volumetry as a novel method to evaluate CW surgery because lung expansion is directly attributed to thoracic deformity and rigidity and affects pulmonary function.
Acknowledgments
This paper was presented in the 27th European Conference on General Thoracic Surgery, June 9-12, 2019, Dublin, Ireland. We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1580/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1580/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1580/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1580/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ito T, Suzuki H, Yoshino I. Mini review: surgical management of primary chest wall tumors. Gen Thorac Cardiovasc Surg 2016;64:707-14. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Isaac KV, Elzinga K, Buchel EW. The Best of Chest Wall Reconstruction: Principles and Clinical Application for Complex Oncologic and Sternal Defects. Plast Reconstr Surg 2022;149:547e-62e. [Crossref] [PubMed]
- Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg 2004;57:295-302. [Crossref] [PubMed]
- Althubaiti G, Butler CE. Abdominal wall and chest wall reconstruction. Plast Reconstr Surg 2014;133:688e-701e. [Crossref] [PubMed]
- Netscher DT, Baumholtz MA. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast Reconstr Surg 2009;124:240e-52e. [Crossref] [PubMed]
- Weiss SW. Histological Typing of Soft Tissue Tumours (WHO. World Health Organization. International Histological Classification of Tumours). 2nd edition ed: Springer-Verlag, 2009.
- Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 2022;52:539-44. [Crossref] [PubMed]
- Yang H, Tantai J, Zhao H. Clinical experience with titanium mesh in reconstruction of massive chest wall defects following oncological resection. J Thorac Dis 2015;7:1227-34. [PubMed]
- Berthet JP, Wihlm JM, Canaud L, et al. The combination of polytetrafluoroethylene mesh and titanium rib implants: an innovative process for reconstructing large full thickness chest wall defects. Eur J Cardiothorac Surg 2012;42:444-53. [Crossref] [PubMed]
- Girotti P, Leo F, Bravi F, et al. The "rib-like" technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg 2011;92:1208-15; discussion 1215-6. [Crossref] [PubMed]
- Marulli G, Hamad AM, Schiavon M, et al. Geometric reconstruction of the right hemi-trunk after resection of giant chondrosarcoma. Ann Thorac Surg 2010;89:306-8. [Crossref] [PubMed]
- Rocco G, Fazioli F, Cerra R, et al. Composite reconstruction with cryopreserved fascia lata, single mandibular titanium plate, and polyglactin mesh after redo surgery and radiation therapy for recurrent chest wall liposarcoma. J Thorac Cardiovasc Surg 2011;141:839-40. [Crossref] [PubMed]
- Rocco G, Mori S, Fazioli F, et al. The use of biomaterials for chest wall reconstruction 30 years after radical surgery and radiation. Ann Thorac Surg 2012;94:e109-10. [Crossref] [PubMed]
- Coonar AS, Qureshi N, Smith I, et al. A novel titanium rib bridge system for chest wall reconstruction. Ann Thorac Surg 2009;87:e46-8. [Crossref] [PubMed]
- Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- De Palma A, Sollitto F, Loizzi D, et al. Chest wall stabilization and reconstruction: short and long-term results 5 years after the introduction of a new titanium plates system. J Thorac Dis 2016;8:490-8. [Crossref] [PubMed]
- Iarussi T, Pardolesi A, Camplese P, et al. Composite chest wall reconstruction using titanium plates and mesh preserves chest wall function. J Thorac Cardiovasc Surg 2010;140:476-7. [Crossref] [PubMed]
- Billè A, Okiror L, Karenovics W, et al. Experience with titanium devices for rib fixation and coverage of chest wall defects. Interact Cardiovasc Thorac Surg 2012;15:588-95. [Crossref] [PubMed]
- Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis 2016;8:S863-71. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- Hayashi T, Sakakura N, Ishimura D, et al. Surgical complication and postoperative pulmonary function in patients undergoing tumor surgery with thoracic wall resection. Oncol Lett 2019;17:3446-56. [Crossref] [PubMed]
- Leuzzi G, Nachira D, Cesario A, et al. Chest wall tumors and prosthetic reconstruction: A comparative analysis on functional outcome. Thorac Cancer 2015;6:247-54. [Crossref] [PubMed]
- Nishida Y, Tsukushi S, Urakawa H, et al. Post-operative pulmonary and shoulder function after sternal reconstruction for patients with chest wall sarcomas. Int J Clin Oncol 2015;20:1218-25. [Crossref] [PubMed]





