
Proteomic investigation and biomarker identification of lung and spleen deficiency syndrome in HIV/AIDS immunological nonresponders
Highlight box
Key findings
• We identified the potential biological markers of typical traditional Chinese medicine (TCM) syndromes in HIV/AIDS immunological nonresponders (INRs).
What is known and what is new?
• Chinese medicine has the ability to promote immune reconstitution in patients in the field of AIDS. However, objective and biological evidence for identifying TCM evidence in HIV/AIDS INRs is still lacking.
• We identified 22 differentially expressed proteins (DEPs) between INRs with lung and spleen deficiency (LSD) syndrome (INRs-LSD) and a healthy control group Through bioinformatics analysis, it was found that these DEPs were mainly associated with the intestinal immune network for immunoglobin A production. Furthermore, we validated the TCM syndrome-specific proteins using enzyme-linked immunosorbent assay.
What is the implication, and what should change now?
• Modern histological techniques should be combined to explore and study the immunological basis of each evidence type and that between the evidence types.
Introduction
Human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) together constitute a major global public health issue. Highly active antiretroviral therapy (HAART) is currently the most effective treatment for suppressing HIV replication and can reduce AIDS-related morbidity and mortality (1). However, some HIV/AIDS patients never experience a rebound in their CD4+ T-cell counts despite the virus not being detected after HAART treatment. These patients are known as HIV/AIDS immune reconstitution-incompetent patients or HIV/AIDS-immunological nonresponders (HIV/AIDS-INRs) (2). Compared to individuals with complete immune reconstitutions, INRs are at a severely impaired stage of immune function and are more likely to develop cardiovascular disease, liver- and kidney-related diseases, and metabolic syndrome or malignancies, leading to a higher mortality rate (3-6). Moreover, a weakened immune system is susceptible to other viruses, and the superimposed infection of multiple viruses can further affect the immune system (7). The mechanisms underlying the development of HIV/AIDS-INRs are complex and influenced by many factors, such as age, gender, genetics, metabolic characteristics, drug effects, timing of HAART, baseline CD4+ T-cell levels, viral load, and cytokines (8). Therefore, the clinical symptoms may differ among INRs, and specific therapeutic plans are needed for individual INRs.
Therefore, HAART currently is faced with several insoluble issues, including immune reconstitution and syndrome-specific treatment, but the unique advantages of traditional Chinese medicine (TCM) may compensate for these deficits in modern care. It has been proven that TCM can alleviate clinical symptoms, improve quality of life, and reduce HAART resistance and toxicity in the field of AIDS (9-11); more importantly, it has been shown capable of promoting the immune reconstruction of HIV/AIDS-INRs (12,13). Moreover, TCM can be tailored to treat the specific syndrome of HIV/AIDS-INRs. TCM syndromes, known as Zheng in Chinese, refers to a process of summarizing and distinguishing comprehensive signals and symptoms of individuals from a particular stage of disease (14). Thus, the acute differentiation of TCM syndromes is the key to guiding the effective prescription of TCM. However, the identification of TCM syndromes in HIV/AIDS-INRs is currently based on traditional inspection, listening and smelling, inquiring, and pulse taking (15). Due to the experience and subjectivity of herbalists, the criteria for diagnosis are often heterogenous. Hence, it is important to objectively define TCM syndrome in HIV/AIDS-INRs. Lung and spleen deficiency (LSD) syndrome is a typical TCM syndrome of HIV/AIDS-INRs, as identified by epidemiological questionnaires applied in our previous work (16), and we sought to turn our research focus to INRs-LSD in the present study. INRs-LSD usually present with symptoms of diarrhea, fever, herpes, mouth sores, skin ulcers, cough, fatigue, and loss of appetite, among others (see supplementary information: Appendix 1).
Proteomics has developed rapidly in recent years and is of great value in medical and therapeutic drug target research (17). Proteomics has been widely used in early diagnosis, prognosis, and monitoring of disease progression, and has made considerable progress in the study of molecular markers for various diseases (18-21), including cancer (22-24). By analyzing the expression of all proteins in an organism, proteomics holistically observes the dynamic changes of proteins and thus has parallels with the holistic concept of TCM. Proteomics has been applied to the investigation of a variety of TCM symptoms, revealing differences in protein expression, structure, function, and interaction between healthy individuals and patients (25). Some scholars have identified urinary protein biomarkers of abdominal allergic purpura using proteomics (26). In this study, tandem mass tag combined with liquid chromatography-tandem mass spectrometry (TMT-LC-MS/MS) was used to screen and identify the differentially expressed proteins (DEPs) in INRs-LSD. These DEPs were then analyzed with bioinformatics assays. Enzyme-linked immunosorbent assay (ELISA) was further conducted to validate the potential biomarkers in order to provide an objective basis for the clinical diagnosis of the syndrome. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-322/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Regional Ethics Review Committee of Sichuan Traditional Chinese Medicine (ethical approval No. 2018KL-062; registration No. ChiCTR1800015290). Informed consent was obtained from all participants.
From September 2018 to April 2020, 29 HIV/AIDS-INRs, including those in the AIDS stage and asymptomatic stage, were enrolled from the Center for Disease Control of Butuo County and Zhaojue County People’s Hospital in Liangshan Prefecture, Sichuan Province, China. These included 19 INRs-LSD (9 participants for TMT-LC-MS/MS proteomics and 10 participants for ELISA analyses), 10 nonsyndromic INRs (NS-INRs), and 20 healthy volunteers.
Diagnostic, inclusion, and exclusion criteria
Diagnostic, inclusion, and exclusion criteria for HIV/AIDS and HIV/AIDS-INR are detailed in the supplemental materials. The diagnostic criteria for the LSD syndrome group were as follows. The primary symptoms included (a) a lack of complexion and the presence of fatigue, low voice, and lethargy in speaking; (b) shortness of breath and asthma, cough with thin sputum, and prolonged cough; and (c) anorexia, bloating, and diarrhea. The secondary symptoms included (a) a pale tongue with white smooth coating and (b) a weak pulse. Patients were diagnosed with LSD syndrome if they met any of the following criteria: (I) with all the primary symptoms; (II) with the primary symptoms of b and c; or (III) with the primary symptoms of the b, c, and any of the secondary symptoms.
Sample collection
Whole blood specimens of peripheral blood (5 mL) were collected from the groups, left at room temperature for 1 h, and centrifuged at 3,500 rpm for 10 min. The supernatant was then aspirated in a lyophilized storage tube and then quickly transferred to a −80 ℃ refrigerator for storage for subsequent analysis.
Tandem mass tag combined with liquid chromatography-tandem mass spectrometry
TMT-labeled reagent was added to each sample peptide, and the reaction was incubated for 1 h at room temperature and then burst with hydroxylamine for 15 min. Subsequently, the TMT-labeled peptides were graded using high-pH reversed-phase high performance liquid chromatography (HPLC). The peptides were eluted in a gradient at a flow rate of 300 nL/min under appropriate conditions, and the effluent was collected. Finally, the samples were fully dissolved and uploaded onto a Q Exactive mass spectrometer (Thermo Fisher Scientific, Massachusetts, USA) to analyze the fractured samples using LC-MS/MS and the full scan range of mass spectra, with the mass to charge ratio (m/z) ranging from 350–1,600. The parent ions were fragmented using a high-energy collision-induced dissociation (HCD) method for secondary mass spectrometry sequence determination and were simultaneously quantified with the ratio of reporter ions. The raw files of the mass spectrometric assays were generated.
Bioinformatics analysis
The differential proteins were screened in the Gene Ontology (GO) database (http://geneontology.org/) and Universal Protein database (https://www.uniprot.org/). The following criteria were used to screen the significant DEPs: a 1% false discovery rate (FDR), fold proteins with fold change greater than or equal to 1.5 or fold change less than or equal to two-thirds, and a P value less than or equal to 0.05. GO annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed on the significant DEPs, the interaction relationships between DEPs were identified using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org/), and the obtained data were imported into Cytoscape software to visualize the interaction network.
ELISA
To further validate the DEPs screened in the LSD syndrome group, at 3 replicates per sample, the serum protein expression levels were measured in INRs-LSD and 10 healthy controls using the human alpha-2-macroglobulin (A2M) ELISA kit (CSB-E08959h) and human selectin L (SELL) ELISA kit (CSB-E04653h) from Wuhan Huamei Bioengineering Co. (Wuhan, China).
Statistical analysis
SPSS 25.0 software (IBM Corp., New York, USA) was used for the statistical analysis of data, and the baseline information of enrolled patients were analyzed using 1-way analysis of variance (ANOVA) and the Mann-Whitney test, with the results of data analysis being expressed as the mean ± standard deviation (SD). P<0.05 was considered a statistically significant difference.
Results
Clinical data statistics
In this study, 19 patients in the LSD syndrome group, 10 patients in the nonsyndromic (NS) group, and 20 people in the healthy control group were included. There was no significant difference in age or sex between the 3 groups (Table S1). There was also no significant difference in HAART hours, viral load, immunological indexes, blood routine, or liver and kidney function indexes in the LSD syndrome group compared with the NS group, although the aspartate aminotransferase level differed (Table S1).
Proteomics analysis
We performed serum proteomics analysis with TMT-LC-MS/MS for both the LSD syndrome group and the NS group. The DEPs were screened at a 1% FDR. A total of 22 DEPs were screened in the LSD syndrome samples, 18 of which were upregulated and 4 downregulated (Table 1, Figure S1). A total of 25 DEPs were screened in the NS samples, 23 of which were upregulated and 2 downregulated (Table 2, Figure S1).
Table 1
| Proteins | Description | Regulation | Score | Coverage | Peptides | FC | P |
|---|---|---|---|---|---|---|---|
| SHBG | Sex hormone-binding globulin isoform 1 precursor [Homo sapiens] | Up | 39.635 | 18% | 7 | 2.5 | 0.00028 |
| PRG2 | Bone marrow proteoglycan isoform 1 preproprotein [Homo sapiens] | Up | 6.699 | 9% | 2 | 1.917 | 0.00234 |
| B2M | Beta-2-microglobulin isoform X1 [Homo sapiens] | Up | 30.83 | 19% | 3 | 1.897 | 0.00970 |
| LOC102723407 | Immunoglobulin heavy variable 4-38-2-like [Homo sapiens] | Up | 29.334 | 14% | 3 | 1.844 | 0.02229 |
| LGALS3BP | Galectin-3-binding protein precursor [Homo sapiens] | Up | 148.961 | 34% | 17 | 1.732 | 0.01897 |
| CETP | Cholesteryl ester transfer protein isoform 1 precursor [Homo sapiens] | Up | 32.095 | 14% | 8 | 1.723 | 0.00000 |
| CHGA | Chromogranin-A isoform 1 preproprotein [Homo sapiens] | Up | 6.282 | 4% | 2 | 1.718 | 0.01067 |
| PIGR | Polymeric immunoglobulin receptor precursor [Homo sapiens] | Up | 41.029 | 15% | 11 | 1.705 | 0.00486 |
| LRG1 | Leucine-rich alpha-2-glycoprotein precursor [Homo sapiens] | Up | 90.859 | 41% | 11 | 1.664 | 0.00123 |
| CD163 | Scavenger receptor cysteine-rich type 1 protein M130 isoform A precursor [Homo sapiens] | Up | 27.698 | 8% | 10 | 1.636 | 0.01080 |
| IGLL5 | Immunoglobulin lambda-like polypeptide 5 isoform 1 [Homo sapiens] | Up | 146.862 | 42% | 12 | 1.635 | 0.02923 |
| FETUB | Fetuin-B isoform 1 precursor [Homo sapiens] | Up | 53.836 | 32% | 12 | 1.631 | 0.00046 |
| SOD2 | Superoxide dismutase [Mn], mitochondrial isoform A precursor [Homo sapiens] | Up | 8.344 | 10% | 2 | 1.609 | 0.01317 |
| VCAM1 | Vascular cell adhesion protein 1 isoform A precursor [Homo sapiens] | Up | 50.798 | 17% | 12 | 1.54 | 0.04096 |
| PI16 | Peptidase inhibitor 16 precursor [Homo sapiens] | Up | 57.646 | 25% | 10 | 1.529 | 0.00482 |
| IGLL1 | Immunoglobulin lambda-like polypeptide 1 isoform C [Homo sapiens] | Up | 27.654 | 27% | 4 | 1.522 | 0.00383 |
| SELL | L-selectin precursor [Homo sapiens] | Up | 48.782 | 17% | 6 | 1.511 | 0.01578 |
| A2M | Alpha-2-macroglobulin isoform X1 [Homo sapiens] | Up | 1208.161 | 64% | 82 | 1.504 | 0.02831 |
| APOA2 | Apolipoprotein A-II preproprotein [Homo sapiens] | Down | 77.946 | 47% | 7 | 0.607 | 0.00225 |
| APOC3 | Apolipoprotein C-III precursor [Homo sapiens] | Down | 62.599 | 59% | 7 | 0.547 | 0.00492 |
| APOA1 | Apolipoprotein A-I isoform 1 preproprotein [Homo sapiens] | Down | 517.655 | 87% | 45 | 0.509 | 0.00351 |
| APOC1 | Apolipoprotein C-I precursor [Homo sapiens] | Down | 61.047 | 40% | 7 | 0.415 | 0.00456 |
DEP, differentially expressed protein; LSD, lung and spleen deficiency; HC, health control; FC, fold change.
Table 2
| Proteins | Description | Regulation | Score | Coverage | Peptides | FC | P |
|---|---|---|---|---|---|---|---|
| PRDX2 | Peroxiredoxin-2 [Homo sapiens] | Up | 29.041 | 28% | 6 | 2.197 | 0.00696 |
| SHBG | Sex hormone-binding globulin isoform 1 precursor [Homo sapiens] | Up | 39.635 | 18% | 6 | 2.11 | 0.00541 |
| CD163 | Scavenger receptor cysteine-rich type 1 protein M130 isoform A precursor [Homo sapiens] | Up | 27.698 | 8% | 10 | 1.961 | 0.00096 |
| PRG2 | Bone marrow proteoglycan isoform 1 preproprotein [Homo sapiens] | Up | 6.699 | 9% | 2 | 1.845 | 0.01603 |
| IGFBP4 | Insulin-like growth factor-binding protein 4 precursor [Homo sapiens] | Up | 9.296 | 14% | 3 | 1.833 | 0.02083 |
| CHGA | Chromogranin-A isoform 1 preproprotein [Homo sapiens] | Up | 6.282 | 4% | 2 | 1.825 | 0.00584 |
| B2M | Beta-2-microglobulin isoform X1 [Homo sapiens] | Up | 30.83 | 19% | 3 | 1.825 | 0.00509 |
| VCAM1 | Vascular cell adhesion protein 1 isoform a precursor [Homo sapiens] | Up | 50.798 | 17% | 12 | 1.76 | 0.01566 |
| VWF | von Willebrand factor preproprotein [Homo sapiens] | Up | 406.585 | 26% | 62 | 1.749 | 0.00982 |
| IGLL5 | Immunoglobulin lambda-like polypeptide 5 isoform 1 [Homo sapiens] | Up | 146.862 | 42% | 12 | 1.711 | 0.04282 |
| LCP1 | Plastin-2 isoform X1 [Homo sapiens] | Up | 8.112 | 4% | 3 | 1.696 | 0.00001 |
| FETUB | Fetuin-B isoform 1 precursor [Homo sapiens] | Up | 53.836 | 32% | 12 | 1.667 | 0.00033 |
| HEG1 | Protein HEG homolog 1 isoform X1 [Homo sapiens] | Up | 13.82 | 2% | 3 | 1.659 | 0.00075 |
| SOD2 | Superoxide dismutase [Mn], mitochondrial isoform A precursor [Homo sapiens] | Up | 8.344 | 10% | 2 | 1.655 | 0.02576 |
| PIGR | Polymeric immunoglobulin receptor precursor [Homo sapiens] | Up | 41.029 | 15% | 11 | 1.645 | 0.01376 |
| HABP2 | Hyaluronan-binding protein 2 isoform 1 preproprotein [Homo sapiens] | Up | 85.367 | 26% | 12 | 1.629 | 0.00013 |
| LRG1 | Leucine-rich alpha-2-glycoprotein precursor [Homo sapiens] | Up | 90.859 | 41% | 11 | 1.608 | 0.00037 |
| SERPINA7 | Thyroxine-binding globulin precursor [Homo sapiens] | Up | 140.116 | 38% | 16 | 1.582 | 0.00094 |
| CP | Ceruloplasmin precursor [Homo sapiens] | Up | 760.474 | 62% | 60 | 1.577 | 0.00211 |
| ALCAM | CD166 antigen isoform 1 precursor [Homo sapiens] | Up | 11.659 | 8% | 4 | 1.571 | 0.01176 |
| CETP | Cholesteryl ester transfer protein isoform 1 precursor [Homo sapiens] | Up | 32.095 | 14% | 8 | 1.565 | 0.00000 |
| LGALS3BP | Galectin-3-binding protein precursor [Homo sapiens] | Up | 148.961 | 34% | 17 | 1.546 | 0.03781 |
| CFP | Properdin precursor [Homo sapiens] | Up | 38.045 | 17% | 7 | 1.546 | 0.02750 |
| APOA1 | Apolipoprotein A-I isoform 1 preproprotein [Homo sapiens] | Down | 517.655 | 87% | 45 | 0.595 | 0.02628 |
| APOC1 | Apolipoprotein C-I precursor [Homo sapiens] | Down | 61.047 | 40% | 7 | 0.415 | 0.00456 |
DEP, differentially expressed protein; NS, nonsyndromic; HC, health control; FC, fold change.
Annotation analysis of the DEPs
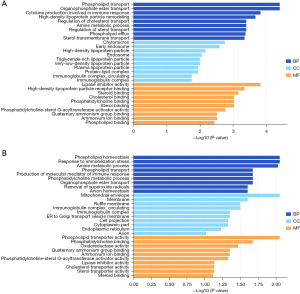
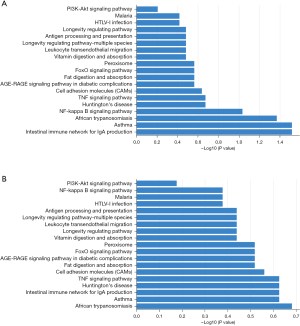
To further clarify the functions of DEPs, we performed bioinformatics analysis of 22 DEPs in the patients with LSD syndrome and 25 DEPs in the NS patients. GO analysis revealed that the DEPs in the LSD syndrome group were mainly localized in early endosome and chylomicron, representing biological processes mainly related to the negative regulation of lipase activity, and the identified molecular functions indicated that the DEPs were mainly involved in lipase inhibitor activity (Figure 1A). The DEPs in the NS patient group were mainly localized in the mitochondrial envelope, representing biological processes mainly related to cytokine production involved in immune response, and the identified molecular functions indicated that the DEPs were mainly involved in phospholipid transporter activity (Figure 1B). KEGG pathway analysis showed that the DEPs in the LSD syndrome groups were mainly enriched in the intestinal immune network for immunoglobin A (IgA) production (hsa04672) (Figure 2A). In contrast, the DEPs in the NS group were mainly enriched in African trypanosomiasis (hsa05143) (Figure 2B).
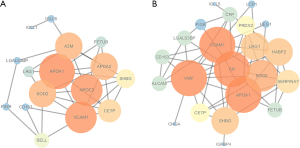
Analysis of protein-protein interaction networks
The interaction relationships between DEPs were identified in the STRING database, and the obtained data were imported into Cytoscape software to visualize the interaction network and construct a protein-protein interaction (PPI) network for these DEPs. We found that most of the DEPs had interactions with each other, and some of the DEPs could show some specific functions in the LSD syndrome group and the NS group (Figure 3). In the LSD syndrome group, apolipoprotein C3 (APOC3), apolipoprotein A2 (APOA2), A2M, SELL, and immunoglobulin lambda-like polypeptide 1 (IGLL1) were at key positions in the PPI. In the NS group, those in key PPI positions were von Willebrand factor (VWF), hyaluronan-binding protein 2 (HABP2), serpin family a member 7 (SERPINA7), and complement factor properdin (CFP). These DEPs were considered to be potential biomarkers for the LSD syndrome group and the NS group.
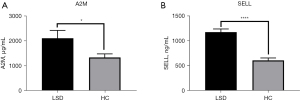
Validation of potential biomarkers with ELISA
The baseline indicators for the LSD syndrome group and the healthy control group are shown in Table S2. By analyzing the enrichment results of DEPs between the LSD syndrome group and the healthy control (HC) group, we found that these DEPs were mainly enriched in the immune system. To validate the TMT-LC-MS/MS results and further identify the biomarkers for INRs-LSD, we selected 2 proteins (A2M and SELL) for validation, as they were the specific DEPs of LSD syndrome and also associated with the immune system. The results showed that both A2M and SELL concentrations were significantly higher in the LSD syndrome group than in the HC group (Figure 4), which was consistent with the results of TMT-LC-MS/MS.
Discussion
For those with AIDS, researchers have tried a variety of treatments, such as human umbilical cord mesenchymal stem cell (MSC) therapy (27), enteral nutritional supplementation (28), and intestinal gut microbial agents (29). As an alternative therapy, TCM can improve the efficacy of HAART for HIV/AIDS and has a high safety profile (30). By analyzing the combined expression of all proteins in an organism, proteomics observes the dynamic changes of proteins through a holistic dimension, which is in line with the holistic concept of TCM (31). Proteomics is now widely used in the determination of TCM syndrome and symptoms to detect differences in protein expression, structure, function, and interactions between healthy individuals and specific syndrome and symptoms, which can provide a scientific and biological basis for TCM syndromes and ultimately reveal the material basis and pathogenesis of diseases (25). The concept of proteomics has also been applied in the field of Chinese medicine research to observe the changes of protein levels in the body through the intervention of Chinese medicine in specific syndromes to reveal the targets of Chinese medicine and to explore the molecular level regulation of the development of syndromes.
In this study, we first compared the differences of routine blood and urine tests, clinical biochemical values, and immunological indexes between the LSD syndrome group and NS group. Subsequently, both the LSD syndrome and NS group in AIDS-INRs were examined with TMT-LC-MS/MS proteomic analysis and comparatively analyzed with bioinformatics. Finally, the specific DEPs of the LSD syndrome group were validated with ELISA, and thus potential biomarkers were identified, with the aim of these potentially providing a biological basis for recognizing TCM syndromes and symptoms in AIDS-INRs. TMT is a widely used technique for quantitative proteomic labeling, which enables the labeling of peptides via binding to the N-terminal group of the peptide and the amino group of the lysine side chain as well as the comparison of proteomes between different samples via labeling different samples with different molecular weight reagents (32). The TMT-LC-MS/MS technique can achieve accurate qualitative and quantitative analysis of proteins in 2 to 10 different samples simultaneously and has the advantages of high detection throughput and high quantitative accuracy (33). Therefore, in this study, we used the TMT-LC-MS/MS technique for proteomic studies. In comparative KEGG pathway analysis, an intestinal immune network for IgA production was more closely associated with LSD than it was with NS. In TCM, there is a mutually effectual interior-exterior relationship between the lung and the large intestine (34). The spleen is the root of acquired nature and regulates the digestion and absorption of nutrients, which is inseparable from the gastrointestinal tract. Within the framework of modern medicine, the gastrointestinal tract is part of the mucosal immune system, and most of the lymphocytes in the gastrointestinal tract can synthesize and secrete immunoglobulin plasma cells, which are the main source of IgA synthesis in the body. Moreover, IgA exerts a protective effect on the integrity of the intestinal barrier, which in turn has an important impact on the elimination of antigens (35). Patients with LSD syndrome often exhibit gastrointestinal symptoms such as diarrhea, abdominal distension, and loss of appetite, which are closely related to the intestinal immune network, and this is consistent with the results of the KEGG pathway analysis in this study.
The ELISA results showed that A2M levels were significantly elevated in patients with LSD syndrome compared to the healthy control group. A2M inhibits broad-spectrum serine and threonine metalloproteinases and inflammatory cytokines and is a major component of the eukaryotic innate immune system (36). It is currently used mainly in neurodegenerative pathologies (37), cerebrovascular diseases (38), inflammation-related diseases (39), and cancer-related immune modulation (40). A2M is a cytokine transporter protein that expresses transforming growth factor-β1 (TGF-β1), which exerts anti-inflammatory activity. Therefore, A2M concentrations increase with increasing protease levels at the site of inflammation (41). Interleukin 10 (IL-10) is an immunomodulatory cytokine with anti-inflammatory activity, and A2M can trigger an anti-inflammatory response by binding to IL-10 (42). INRs-LSD are often accompanied by a series of chronic inflammatory responses, such as prolonged diarrhea, cough, and low fever. Upregulated A2M often suggests an inflammatory response in vivo, which is consistent with the upregulation of A2M expression observed in this study. Among the participants examined, LSD-INRs had lower platelet levels compared to NS-INRs, which may also suggest the presence of inflammation in vivo. Thymosin can enhance cellular immune function and regulate immune homeostasis, and under normal conditions, thymosin exerts its immune effects through binding to zinc (43). While A2M has a higher binding affinity for zinc than does thymosin, it has been shown that in patients with cervical cancer, there is reduced peripheral zinc bioavailability, reduced plasma active thymosin level, reduced natural killer cell activity, reduced IL-2 level, and increased A2M level, suggesting that increased A2M competing with thymosin for zinc binding may lead deficiency and impaired immune function in vivo (44). Since A2M has a high binding capacity for thymosin zinc, it may play a key role in immune efficiency. In this study, A2M was upregulated in patients in the LSD syndrome group, suggesting impaired immune efficiency in vivo, which is consistent with the characteristics of AIDS-INRs.
According to ELISA, SELL levels were also significantly higher in the LSD syndrome group compared to the HCs. SELL, or L-selectin, is a type I transmembrane glycoprotein and cell adhesion molecule that is a member of the selectin family, can be expressed on most leukocytes, and is a major regulator of leukocyte adhesion, migration, and signaling (45). Currently, SELL is most commonly used for inducing T-lymphocyte homing and has many antitumor applications (46). Chronic inflammation may promote tumor growth through myeloid-derived suppressor cells (MDSCs) to induce SELL expression and promote metastasis of the tumors to the lymph nodes (47). MDSCs can use multiple mechanisms to block innate and adaptive antitumor immunity and suppress CD4+ T lymphocytes and CD8+ T lymphocytes (48). One study showed that MDSC levels in peripheral blood of patients with HIV/AIDS with lung spleen qi deficiency syndrome were negatively correlated with CD4+ T-cell levels, suggesting that MDSC may affect immune function in AIDS by regulating SELL expression (49). The population targeted in this study was HIV/AIDS-INRs, who are at a higher risk of opportunistic infections and are more likely to be associated with the incidence of tumors and other diseases than are those with HIV/AIDS. Furthermore, LSD syndrome is inherently a qi deficiency, which is associated with clinical symptoms of qi and blood deficiency, the inability to produce blood, and even worse immune function. In this study, we found that SELL was upregulated in AIDS-INRs with LSD syndrome compared with the HC group, suggesting that SELL is upregulated when immune deficiency is present. Thus, SELL may serve as a target for the treatment of HIV/AIDS and the reconstitution of immune status, possibly through the downregulation of its expression.
Conclusions
Based on the DEPs discovered with proteomics and on bioinformatics analyses, it was found that there were biological differences between HCs and HIV/AIDS-INRs with the typical TCM syndrome of LSD. Furthermore, TCM syndrome-specific DEPs including A2M and SELL were identified as the serum biomarkers for LSD syndrome in INRs. These findings may help contribute to the scientific and objective evidence for the differentiation and clinical diagnosis of LSD syndrome in INRs, providing a biological basis for a deeper understanding of TCM syndromes and more opportunities for the stable application TCM’s strengths in the treatment of HIV/AIDS-INRs. Due to the small number of cases included, which is a limitation this study, we will further expand the number of cases for analysis in follow-up research.
Acknowledgments
We thank all the blood donors from the Center for Disease Control of Butuo County and Zhaojue County People’s Hospital in Liangshan Prefecture (Sichuan, China).
Funding: This study was supported by the National Major Science and Technology Projects of China (No. 2017ZX10205501), the Science and Technology Project of Sichuan Province (No. 2020YFS0333), the National Natural Science Foundation of China (No. 82104569), the Applied Basic Research Project of Sichuan Provincial Science and Technology Department (No. 2021YJ0253), and the Chengdu University of Traditional Chinese Medicine Foundation (Nos. ZKYY2003 and QJRC2022002).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-322/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-322/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-322/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-322/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Regional Ethics Review Committee of Sichuan Traditional Chinese Medicine, China (ethical approval No. 2018KL-062). Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003;362:22-9. [Crossref] [PubMed]
- Battegay M, Nüesch R, Hirschel B, et al. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006;6:280-7. [Crossref] [PubMed]
- Baker JV, Peng G, Rapkin J, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr 2008;48:541-6. [Crossref] [PubMed]
- Pacheco YM, Jarrin I, Rosado I, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res 2015;117:69-74. [Crossref] [PubMed]
- Takuva S, Maskew M, Brennan AT, et al. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Soc 2014;17:18651. [Crossref] [PubMed]
- Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014;58:1312-21. [Crossref] [PubMed]
- Zhao M, Zhuo C, Li Q, et al. Cytomegalovirus (CMV) infection in HIV/AIDS patients and diagnostic values of CMV-DNA detection across different sample types. Ann Palliat Med 2020;9:2710-5. [Crossref] [PubMed]
- Yang X, Su B, Zhang X, et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J Leukoc Biol 2020;107:597-612. [Crossref] [PubMed]
- Zou W, Wang J, Liu Y. Effect of traditional Chinese medicine for treating human immunodeficiency virus infections and acquired immune deficiency syndrome: Boosting immune and alleviating symptoms. Chin J Integr Med 2016;22:3-8. [Crossref] [PubMed]
- Xu LR, Yang XP, Guo HJ, et al. Study on quality of life of asymptomatic HIV infected persons with traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2013;38:2480-3. [PubMed]
- Jiang SQ, Sun HX, Xu YM, et al. Effects of jingyuankang capsules on leukocyte level in AIDS patients. J Tradit Chin Med 2011;31:32-5. [Crossref] [PubMed]
- Wang J, Liang B, Zhang X, et al. An 84-month observational study of the changes in CD4 T-lymphocyte cell count of 110 HIV/AIDS patients treated with traditional Chinese medicine. Front Med 2014;8:362-7. [Crossref] [PubMed]
- Wang J, Li Y, Tang YL, et al. Effect of Immune No. 2 on the immune reconstitution in patients with HIV/AIDS after highly active antiretroviral treatment: a randomized double blind placebo controlled clinical trial. Chin J Integr Med 2013;19:340-6. [Crossref] [PubMed]
- Wen L, Liu YF, Jiang C, et al. Comparative Proteomic Profiling and Biomarker Identification of Traditional Chinese Medicine-Based HIV/AIDS Syndromes. Sci Rep 2018;8:4187. [Crossref] [PubMed]
- Dai J, Sun S, Cao H, et al. Applications of New Technologies and New Methods in ZHENG Differentiation. Evid Based Complement Alternat Med 2012;2012:298014. [Crossref] [PubMed]
- Ji SX, Zheng YF, Li X, et al. Epidemiological investigation and proteomic profiling of typical TCM syndrome in HIV/AIDS immunological nonresponders. Anat Rec (Hoboken) 2022; Epub ahead of print. [Crossref] [PubMed]
- Cifani P, Kentsis A. Towards comprehensive and quantitative proteomics for diagnosis and therapy of human disease. Proteomics 2017; [Crossref] [PubMed]
- Moresco RN, De Carvalho JAM. Applying proteomics to diagnosis of diabetic kidney disease. Expert Rev Proteomics 2017;14:841-3. [Crossref] [PubMed]
- Yang J, Yang L, Li B, et al. iTRAQ-Based Proteomics Identification of Serum Biomarkers of Two Chronic Hepatitis B Subtypes Diagnosed by Traditional Chinese Medicine. Biomed Res Int 2016;2016:3290260. [Crossref] [PubMed]
- Insenser M, Escobar-Morreale HF. Proteomics and polycystic ovary syndrome. Expert Rev Proteomics 2013;10:435-47. [Crossref] [PubMed]
- Chen Y, Huang A, Ao W, et al. Proteomic analysis of serum proteins from HIV/AIDS patients with Talaromyces marneffei infection by TMT labeling-based quantitative proteomics. Clin Proteomics 2018;15:40. [Crossref] [PubMed]
- Tan HT, Lee YH, Chung MC. Cancer proteomics. Mass Spectrom Rev 2012;31:583-605. [Crossref] [PubMed]
- Balestrieri ML, Giovane A, Mancini FP, et al. Proteomics and cardiovascular disease: an update. Curr Med Chem 2008;15:555-72. [Crossref] [PubMed]
- Huang Z, Ma L, Huang C, et al. Proteomic profiling of human plasma for cancer biomarker discovery. Proteomics 2017; [Crossref] [PubMed]
- Lu CL, Qv XY, Jiang JG. Proteomics and syndrome of Chinese medicine. J Cell Mol Med 2010;14:2721-8. [Crossref] [PubMed]
- Jia L, Wu J, Wei J, et al. Proteomic analysis of urine reveals biomarkers for the diagnosis and phenotyping of abdominal-type Henoch-Schonlein purpura. Transl Pediatr 2021;10:510-24. [Crossref] [PubMed]
- Zhang Z, Fu J, Xu X, et al. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS 2013;27:1283-93. [Crossref] [PubMed]
- Geng ST, Zhang JB, Wang YX, et al. Pre-Digested Protein Enteral Nutritional Supplementation Enhances Recovery of CD4(+) T Cells and Repair of Intestinal Barrier in HIV-Infected Immunological Non-Responders. Front Immunol 2021;12:757935. [Crossref] [PubMed]
- Lyu W, Meng Q, Xiao J, et al. Gut lactate-producing bacteria promote CD4 T cell recovery on Anti-retroviral therapy in HIV-infected patients. Comput Struct Biotechnol J 2021;19:2928-37. [Crossref] [PubMed]
- Qian Z, Zhang Y, Xie X, et al. Efficacy and safety of traditional Chinese herbal medicine combined with HAART in the treatment of HIV/AIDS: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e28287. [Crossref] [PubMed]
- Li X, Wang W, Chen J. Recent progress in mass spectrometry proteomics for biomedical research. Sci China Life Sci 2017;60:1093-113. [Crossref] [PubMed]
- Anderson NL, Matheson AD, Steiner S. Proteomics: applications in basic and applied biology. Curr Opin Biotechnol 2000;11:408-12. [Crossref] [PubMed]
- Jiang X, Bomgarden R, Brown J, et al. Sensitive and Accurate Quantitation of Phosphopeptides Using TMT Isobaric Labeling Technique. J Proteome Res 2017;16:4244-52. [Crossref] [PubMed]
- Tang J, Xu L, Zeng Y, et al. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol 2021;91:107272. [Crossref] [PubMed]
- Gommerman JL, Rojas OL, Fritz JH. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 2014;5:652-62. [Crossref] [PubMed]
- Wong SG, Dessen A. Structure of a bacterial alpha2-macroglobulin reveals mimicry of eukaryotic innate immunity. Nat Commun 2014;5:4917. [Crossref] [PubMed]
- Mocchegiani E, Malavolta M. Zinc dyshomeostasis, ageing and neurodegeneration: implications of A2M and inflammatory gene polymorphisms. J Alzheimers Dis 2007;12:101-9. [Crossref] [PubMed]
- Yamada M, Sodeyama N, Itoh Y, et al. A deletion polymorphism of alpha(2)-macroglobulin gene and cerebral amyloid angiopathy. Stroke 1999;30:2277-9. [Crossref] [PubMed]
- Wang T, Wang X, Yao Y, et al. Association of plasma apolipoproteins and levels of inflammation-related factors with different stages of Alzheimer's disease: a cross-sectional study. BMJ Open 2022;12:e054347. [Crossref] [PubMed]
- Fang K, Caixia H, Xiufen Z, et al. Screening of a Novel Upregulated lncRNA, A2M-AS1, That Promotes Invasion and Migration and Signifies Poor Prognosis in Breast Cancer. Biomed Res Int 2020;2020:9747826. [Crossref] [PubMed]
- Garber TR, Gonias SL, Webb DJ. Interleukin-4 and IL-10 bind covalently to activated human alpha2-macroglobulin by a mechanism that requires Cys949. J Interferon Cytokine Res 2000;20:125-31. [Crossref] [PubMed]
- Acuner-Ozbabacan ES, Engin BH, Guven-Maiorov E, et al. The structural network of Interleukin-10 and its implications in inflammation and cancer. BMC Genomics 2014;15:S2. [Crossref] [PubMed]
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 2022;35:187-213. [Crossref] [PubMed]
- Mocchegiani E, Ciavattini A, Santarelli L, et al. Role of zinc and alpha2 macroglobulin on thymic endocrine activity and on peripheral immune efficiency (natural killer activity and interleukin 2) in cervical carcinoma. Br J Cancer 1999;79:244-50. [Crossref] [PubMed]
- Ivetic A, Hoskins Green HL, Hart SJ. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front Immunol 2019;10:1068. [Crossref] [PubMed]
- Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel) 2016;8:36. [Crossref] [PubMed]
- Perfilyeva YV, Abdolla N, Ostapchuk YO, et al. Chronic Inflammation Contributes to Tumor Growth: Possible Role of L-Selectin-Expressing Myeloid-Derived Suppressor Cells (MDSCs). Inflammation 2019;42:276-89. [Crossref] [PubMed]
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 2010;59:1593-600. [Crossref] [PubMed]
- Liu Z, Deng BW, Li CC, et al. Changes of Treg and MDSCs in peripheral blood of HIV/AIDS patients with syndrome of qi deficiency of lung and spleen. China Journal of Traditional Chinese Medicine and Pharmacy 2021;36:2901-3.
(English Language Editor: J. Gray)


