
Improved diagnostic yield of symptom association probability involving only cough for gastroesophageal reflux-induced chronic cough
Highlight box
Key findings
• Symptom association probability involving only cough (C-SAP) was superior to that involving total symptoms (T-SAP) for the diagnosis of gastroesophageal reflux-induced chronic cough (GERC), and its use might improve diagnostic efficacy.
What is known and what is new?
• A positive SAP was recommended for identifying GERC but with a low sensitivity. The calculation of SAP includes both C-SAP and T-SAP in clinical practice.
• Which SAP is superior and more suitable for the diagnosis of GERC and prediction of therapeutic efficacy needs the assessment.
What is the implication, and what should change now?
• C-SAP might be a more precise criterion for GERC diagnosis and could be used to replace T-SAP, even though its efficacy was still suboptimal.
Introduction
Gastroesophageal reflux-induced chronic cough (GERC) is a specific type of gastroesophageal reflux disease (GERD) characterized by a predominant cough and is a common cause of chronic cough (1-4). When diagnosing GERC, it is important to obtain objective evidence of abnormal reflux and establish the assumed cause-effect relationship of the reflux-cough sequence. Multichannel intraluminal impedance and pH monitoring (MII-pH) is used to assess acid and non-acid reflux episodes, as well as their temporal association with cough (5). Thus, MII-pH is recommended as the most useful laboratory investigation for GERC diagnosis (6,7).
According to the Lyon Consensus, esophageal acid exposure time (AET), symptom association probability (SAP), and the number of reflux episodes are the main MII-pH metrics used for identifying GERC (5). The SAP is a measure that uses statistical calculations to express the probability that a truly significant symptom-reflux relationship does not exist by chance, and it is considered positive at ≥95% (8,9). The current Chinese guideline for cough management recommends a positive SAP as one of the diagnostic criteria for GERC (10). However, the calculation process of SAP has not been standardized yet.
There are two approaches for SAP calculation: taking all the recorded reflux-related symptoms including cough into account or considering only cough as a relevant component (11-14). While the former is widely employed for the diagnosis of GERD (5), the latter has only been used for determining the relationship between reflux and chronic cough in several studies and has not been applied for GERC identification in clinical practice (11,14). Which SAP is superior and more suitable for GERC diagnosis remains to be elucidated.
Therefore, we conducted a prospective clinical investigation to compare the efficacies of SAPs calculated by the above two methods in identifying GERC and tried to improve the diagnostic yield of GERC. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1016/rc).
Methods
Patients
Consecutive patients referred to our respiratory clinic for evaluation of potential GERC were enrolled between January 2017 and May 2021. Eligible inclusion criteria for participation were as follows: (I) aged between 18 and 70 years old; (II) cough lasting for >8 weeks, with concomitant other reflux-related symptoms such as regurgitation and heartburn; (III) other possible causes of chronic cough including cough variant asthma, upper airway cough syndrome, and eosinophilic bronchitis were excluded after a sequential laboratory work-up; and (IV) successful MII-pH. The exclusion criteria were as follows: (I) other co-existing causes of chronic cough; (II) neither cough events nor other reflux-related symptoms were reported, or fewer than four cough events were recorded during MII-pH; or (III) patients had incomplete clinical information or were lost to follow-up.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Tongji Hospital (No. LL(H)-2016-396) and registered as ChiCTR-DDD-17012587 in the Chinese Clinical Trials Registry (http://www.chictr.org/). Written informed consent was obtained from all participants before enrollment.
MII-pH
MII-pH was performed when subjects had been off acid suppression treatment for at least 1 week as described previously (15). Briefly, a MII-pH catheter containing an antimony pH probe (819100, Medical Measurement System B.V., Enschede, Netherlands) and six impedance channel amplifiers (K6011-E10632, Unisensor, Seraing, Belgium) was transnasally inserted into the esophagus. The pH electrode was positioned 5 cm and impedance channel amplifiers were located at 3, 5, 7, 9, 15, and 17 cm above the proximal border of lower esophageal sphincter as determined by high-resolution manometry just prior to MII-pH. The signals were collected from all seven channels with 50 Hz frequency over 24 h using a portable digital data logger (Ohmega, Medical Measurement System B.V.) connected to the MII-pH catheter. The patients were instructed to maintain their normal daily life and push an event marker on the digital data logger whenever cough or other reflux-related symptoms occurred. They were also asked to keep a journal to record the timing of meals, recumbent position, and symptom episodes in detail. These data were used to check the appropriate use of the event button and distinguish the type of symptoms. No significant adverse events occurred during the above tests.
The downloaded data were automatically analyzed and manually reviewed using specific software (Database soft, 8.7 version, Medical Measurement System B.V.). The reflux episodes were classified as liquid, gas, or mixed reflux based on the impedance values, and categorized into acidic (pH <4.0), weakly acidic (pH 4.0–7.0) and weakly alkaline reflux (pH >7.0), with the latter two defined as non-acidic reflux (16). AET and the number of reflux episodes were also analyzed.
The SAP was calculated to determine the temporal association between symptoms recorded in the journal and MII-detected reflux (including acidic and non-acidic reflux) according to the protocol described previously (12,17). The entire recording of MII-pH was divided into consecutive 2-min time windows and assessed for the presence of reflux in each time window. In combination with symptom time and the number of all the symptoms recorded in the journals, each 2-min period of the recording was classified into reflux alone (R+S−), reflux followed by symptom (R+S+), only symptom but no reflux (R−S+), and neither reflux nor symptom (R−S−). When a symptom occurred in the 2-min span after a reflux event, it was considered as “associated to reflux”. A contingency table was constructed for each patient containing the numbers of the different types of 2-min windows using Fisher’s exact test, appropriately adjusted where low numbers occurred in one of the four fields. The probability (P value) was then calculated to determine if the observed association between reflux and symptom occurred by chance, and the SAP was expressed as (1.0–P)×100%. An SAP value ≥95% was considered positive.
For comparison between the two calculation methods, SAPs in each patient were independently re-analyzed by only reviewing the recordings of cough episodes (not the other reflux-related symptoms such as regurgitation and heartburn) marked in the journal during MII-pH, and these were labeled as C-SAP. The SAP involving the total symptoms (cough and all the other reflux-related symptoms) was termed T-SAP. The SAPs for acidic and non-acidic reflux events were also calculated for individual patients based on the pH of the refluxates, reflecting the likelihood of acidic and non-acidic reflux as the cough cause, respectively.
Study procedures
This was a single-center observational study. Initial patient assessment included the collection of general information, cough severity recording, and MII-pH testing. Cough severity was scored using the validated Chinese version (18) of the cough symptom score developed by Hsu et al. (19), which rates daytime and nighttime cough from 0 (no coughing) to 5 (most intense coughing). Regardless of the MII-pH results, all patients with potential GERC received an 8-week of standard anti-reflux therapy comprising omeprazole (20 mg twice daily 30 min before meals) and domperidone (10 mg three times daily), followed by an intensified anti-reflux trial that included an increase in the omeprazole dose to 80 mg daily or add-on therapy with baclofen or gabapentin when the initial treatment failed and refractory GERC was suspected (20). The patients were followed up every 2 weeks, and their responses to therapy and cough symptom score were rated each time. GERC was definitively confirmed when cough was completely resolved or significantly improved (cough symptom score decreased by >50%) in response to the anti-reflux therapy, in addition to objective evidence of abnormal gastroesophageal reflux defined as any AET >6%, SAP ≥95%, or >80 reflux episodes/24 h. Patients with abnormal acidic and non-acidic reflux were diagnosed with acid GERC and non-acid GERC, respectively (16). When cough failed to improve after all the anti-reflux trials, empirical therapy targeting the other causes such as cough variant asthma, upper airway cough syndrome, eosinophilic bronchitis and atopic cough was performed even though the initial negative relevant laboratory findings. The diagnosis was considered as probable if a favorable response to the therapy specific to the etiology was observed (21).
Statistical analysis
According to our published observations (6,22) and the literature data (11,13,14), prospective statistical power calculation indicated that the minimum of 103 patients would be required to provide 80% power to detect a 25% difference in sensitivity between C-SAP and T-SAP using a 5% two-sided test. In addition to the predicted 6% dropout or lost follow-up, the final recruited numbers were 110 patients.
Data with normal distributions are expressed as mean ± SD, whereas those with skewed distribution are expressed as medians (25%, 75% interquartile range). Receiver operating characteristic (ROC) curve analysis was performed to determine the efficacies of SAPs in diagnosing GERC. Student’s t-tests, Mann-Whitney tests, χ2 tests, DeLong’s tests, and Kappa tests were used for the comparisons between groups where applicable. SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Differences were considered significant at P<0.05.
Results
General information
A total of 213 patients with suspected GERC who underwent MII-pH met the recruitment criteria during the research period. Ninety-eight subjects were excluded because of cough reported alone (n=69), fewer than four cough episodes (n=12), no symptom recording (n=10), and only other reflux-related symptoms without cough bursts (n=7) during MII-pH. Ten other patients were excluded due to incomplete MII-pH recordings (n=5), failure to completion of anti-reflux therapy course (n=3), and loss to the follow-up (n=2). Finally, 105 patients were included in the analysis. The study flow diagram and general patient characteristics are shown in Figure 1 and Table 1.

Table 1
| Characteristic | Value |
|---|---|
| Male/female (n) | 45/60 |
| Age (year), mean ± SD | 46.0±14.9 |
| Cough duration (month), median [IQR] | 12 [6–36] |
| Cough symptom score, median [IQR] | |
| Daytime | 3 [3–4] |
| Nighttime | 1 [1–2] |
| Number of coughs, median [IQR] | 9 [6–18] |
| Number of reflux episodes, median [IQR] | 83 [50–113] |
| Esophageal acid exposure time, mean ± SD | 4.73±12.29 |
| Pulmonary function test, mean ± SD | |
| FEV1 (% predictive value) | 97.16±14.54 |
| FVC (% predicted) | 122.69±149.81 |
| FEV1/FVC (%) | 82.00±9.50 |
SD, standard deviation; IQR, interquartile range; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Sixty-five (61.9%) patients favorably responded to anti-reflux treatment and were definitively diagnosed with GERC, including 27 (41.5%) cases of acid GERC and 38 (58.5%) cases of non-acid GERC. For therapeutic response of GERC, standard anti-reflux treatment was effective in 22 (33.8%) patients while subsequent intensified anti-reflux therapy benefited the other 43 (66.2%) patients, including omeprazole dose increased to 80 mg daily in 11, add-on therapy with baclofen in 7 and with gabapentin in 25. Intensified anti-reflux treatment was more frequently needed in non-acid GERC (30/38) than in acid GERC (13/27) for cough resolution (78.9% vs. 48.1%, χ2=6.687, P=0.01). Among 40 non-responders, their cough could be explained by cough variant asthma (n=12), upper-airway cough syndrome (n=8), eosinophilic bronchitis (n=7), atopic cough (n=5), and unknown causes (n=8) based on the therapeutic response to subsequent empirical treatment.
Symptoms and SAP distributions
The distribution of symptoms recorded by patients during MII-pH is shown in Figure 2A. All participants reported cough as their predominant symptom. Regurgitation was present in 69 of 105 (65.7%) patients, while heartburn was reported in 47 (44.8%) patients. There was a wide distribution of SAPs that ranged from 0% to 100%, but most concentrated on the 0 or between 50–100% for both C-SAP and T-SAP (Figure 2B).
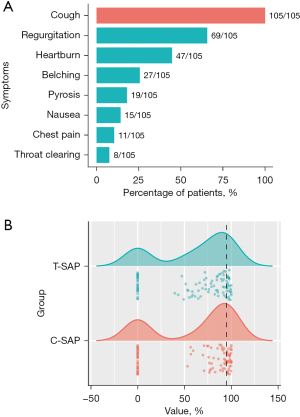
Comparison of C-SAP with T-SAP in identifying GERC
In 105 patients, the positive C-SAP and T-SAP rates were 34.3% (36 cases) and 23.8% (25 patients), respectively (Figure 1). No statistically significant difference was found between them (P>0.05). GERC was confirmed in 35 of 36 (97.2%) patients with a positive C-SAP and 22 of 25 (88.0%) patients with a positive T-SAP. C-SAP had a higher sensitivity, positive predictive value, accuracy, Youden index, and area under the curve (AUC) than T-SAP for the identification of GERC (Table 2). Both had high and comparable specificities (97.5% vs. 92.5%, χ2=5.556, P=0.121). Their negative predictive values were low and similar (Table 2).
Table 2
| Items | C-SAP | T-SAP | χ2 value | P value |
|---|---|---|---|---|
| AUC | 0.757 | 0.632 | – | <0.001 |
| Youden index | 0.513 | 0.263 | – | – |
| Sensitivity, % | 53.85 | 33.85 | 8.117 | 0.004 |
| Specificity, % | 97.50 | 92.50 | 5.556 | 0.121 |
| PPV, % | 97.22 | 88.00 | 5.838 | 0.016 |
| NPV, % | 56.52 | 46.25 | 2.422 | 0.120 |
| Accuracy, % | 70.48 | 56.19 | 4.204 | 0.040 |
| κ value | 0.451 | 0.221 | – | – |
| χ2 value | 28.975 | 9.475 | – | – |
| P value | <0.001 | 0.002 | – | – |
SAP, symptom association probability; GERC, gastroesophageal reflux-induced chronic cough; C-SAP, SAP calculated with only cough; T-SAP, SAP calculated with total symptoms; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
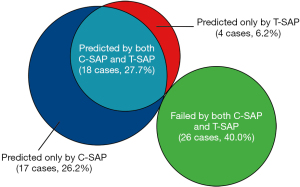
For the identification of GERC, there was a 27.7% (18 cases) overlap between SAPs. Additionally, C-SAP and T-SAP exclusively predicted 26.2% (17 cases) and 6.2% (4 cases) of GERC, respectively. Approximately 40.0% (26 cases) of GERC cases were not predicted by either SAP (Figure 3).
C-SAP and T-SAP for acid and non-acid GERC
A sub-analysis showed that C-SAP had a distinctly higher sensitivity than T-SAP in identifying both acid and non-acid GERC. Furthermore, the AUC was significantly larger for C-SAP than T-SAP for the diagnosis of non-acid GERC. However, the other ROC analysis metrics were similar between C-SAP and T-SAP (Table 3).
Table 3
| Items | SAPs for acid GERC | SAPs for non-acid GERC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C-SAP | T-SAP | χ2 value | P value | C-SAP | T-SAP | χ2 value | P value | ||
| AUC | 0.746 | 0.660 | – | 0.072 | 0.792 | 0.653 | – | 0.013 | |
| Youden index | 0.493 | 0.284 | – | – | 0.583 | 0.294 | – | – | |
| Sensitivity, % | 51.85 | 33.33 | 7.386 | 0.007 | 65.79 | 39.47 | 14.617 | <0.001 | |
| Specificity, % | 97.44 | 98.72 | 1.020 | 0.312 | 92.54 | 91.04 | 0.272 | 0.602 | |
| PPV, % | 87.50 | 90.00 | 0.409 | 0.523 | 83.33 | 71.43 | 4.065 | 0.044 | |
| NPV, % | 85.39 | 81.05 | 0.567 | 0.451 | 82.67 | 72.62 | 2.914 | 0.088 | |
| Accuracy, % | 85.71 | 81.90 | 0.595 | 0.440 | 82.86 | 72.38 | 3.470 | 0.063 | |
| χ2 value | 37.724 | 23.912 | – | – | 40.420 | 14.115 | – | – | |
| κ value | 0.569 | 0.404 | – | – | 0.611 | 0.338 | – | – | |
| P value | <0.001 | <0.001 | – | – | <0.001 | <0.001 | – | – | |
SAP, symptom association probability; GERC, gastroesophageal reflux-induced chronic cough; C-SAP, SAP calculated with only cough; T-SAP, SAP calculated with total symptoms; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
Comparison between GERC patients with positive and negative C-SAPs
In 65 GERC patients, the positive C-SAP group accounted for 53.8% (35 cases) while the negative C-SAP group accounted for 46.2% (30 cases). More GERC patients (29/35) with positive C-SAP were resistant to standard anti-reflux treatment and needed the intensified anti-reflux therapy to resolve their cough when compared with those (14/30) with negative C-SAP (82.9% vs. 46.7%, χ2=9.449, P=0.002). However, there was no significant difference in the other clinical characteristics such as age and gender distribution, cough duration or score and MII-pH variables between GERC patients with positive and negative C-SAPs (data are not shown).
Discussion
The SAP analysis seems to be an appropriate method to precisely document the temporal association between reflux events and symptom episodes during MII-pH (5). It can be calculated by selecting either cough alone or all the reflux-related symptoms (12). Nevertheless, few international cough guidelines have attempted to develop a uniform standard calculation method of SAP for GERC (8,11-13,17,23). Significant concerns were raised about the validity of clinical decision-making based on a positive SAP. In the present study, we assessed the diagnostic accuracy of C-SAP (cough only) and T-SAP (total symptoms involved) in a cohort of suspected GERC patients with both cough and other reflux-related symptoms. GERC was definitively confirmed by a favorable response to a standard or intensified medical anti-reflux therapy. Our results demonstrate that both C-SAP and T-SAP had moderate abilities to identify GERC with high and comparable specificities. C-SAP was superior to T-SAP since it achieved a higher diagnostic yield for GERC, as evidenced by a distinctly higher sensitivity, positive predictive value, accuracy, Youden index, and larger AUC. C-SAP was also more sensitive than T-SAP in predicting both acid and non-acid GERC. These findings indicate that C-SAP may be a more precise criterion for GERC diagnosis, and its use could improve diagnostic efficacy.
The reliability of SAP is critically dependent on the reliable execution of the reflux monitoring procedure and accurate analysis protocols, including careful selection of symptoms of interest (5). A previous study showed that 43.2% of patients with a positive SAP were observed for reflux-related cough, while SAP for other reflux-related symptoms were positive in 44.0% of patients, and only 10.8% of patients had a positive SAP for both other reflux-related symptoms and cough (13). This suggests that other reflux-related symptoms such as regurgitation and heartburn are not necessarily valuable when attempting to determine the temporal association between reflux and cough. In keeping with this observation, we found a higher sensitivity for GERC identification with C-SAP compared to T-SAP. A likely explanation for this is that cough, as an extra-esophageal symptom of GERD, may be induced either by reflux or by non-reflux causes. In the patients with GERC diagnosed by a positive T-SAP, it is possible that regurgitation and heartburn resolved, whereas cough failed to improve after the initiation of consequent anti-reflux therapy (24). It was originally believed that cough was less responsive to anti-reflux treatment than other reflux-related symptoms (25), which can be defined as refractory GERC (26). Actually, unlike regurgitation and heartburn, cough itself was probably irrelevant to reflux episodes, and C-SAP may more robustly establish reflux-cough causality.
Accumulating evidence suggests that a positive SAP is an independent predictor for a favorable response to anti-reflux treatment in patients with chronic cough (5,27,28). Theoretically, C-SAP should be expected to have a lower sensitivity and higher specificity for GERC identification when the other reflux-related symptoms were removed from the calculation because there would be an obvious decrease in the number of total marked symptoms. It is therefore surprising that the sensitivity of C-SAP was higher than that of T-SAP without affecting specificity. Actually, C-SAP showed slightly higher specificity for GERC diagnosis than T-SAP, but the difference did not reach statistical significance. Since the rate of positive SAPs mostly depends on the symptoms that occur within the 2-min time windows of MII-pH records containing a reflux event, the percentage of reflux-related symptoms rather than the absolute number of all symptoms matters for SAP calculations (11). In the context of GERC, cough is induced by reflux and is more prone to fall within the 2-min time window, thus leading to a higher sensitivity of C-SAP. Our results provide additional evidence that SAP is an effective indicator for GERC prediction and support replacing T-SAP with C-SAP in the identification of GERC.
Both non-acid and acidic reflux events were associated with cough (5,12,15). Like acidic reflux in acid GERC, non-acidic reflux was mainly responsible for non-acid GERC (15). When acid and non-acid reflux events were considered separately, more acid and non-acid GERC (especially a higher percentage of non-acid GERC) were identified by C-SAP than by T-SAP, suggesting that concomitant other reflux-related symptoms were not good predictors for acid or non-acid GERC. The reason why non-acid GERC was more likely to be diagnosed by C-SAP than T-SAP may be explained by the recruited patients; we selected a larger number of patients with non-acid GERC who might record fewer other reflux-related symptoms but more cough events than those with acid GERC. We previously demonstrated that non-acid GERC had less frequent regurgitation and heartburn than acid GERC (22), and weakly acidic reflux was mainly related to cough hypersensitivity in non-acid GERC (15). When T-SAP alone was used to diagnose GERC, it had a roughly equal ability to identify both acid and non-acid GERC, which was consistent with our previous study (6). These findings revealed that refluxate acidity was not the only factor involved in the reflux-induced cough, and C-SAP might be more useful for identifying both acid and non-acid GERC.
Besides its more efficient identification for potential GERC, a positive C-SAP may predict the necessity and success of intensified anti-reflux trial, as indicated by the data regarding C-SAP in the study. It is clinically significant and important. In real-world practice, chronic cough with concomitant other reflux-related symptoms in patients leads generally to an empirical trial of proton pump inhibitors (4). If it fails or the reflux-related symptoms other than cough are absent, MII-pH is often ordered to examine the etiology underlying chronic cough, especially when patients are referred to cough specialists since easy to treat causes have usually been eliminated (6). With a positive C-SAP alone or in combination with pathological AET by MII-pH, one can be sure of GERC diagnosis and initiate the medical anti-reflux therapy immediately or become confident to implement the intensified anti-reflux treatment when the previous trial with proton pump inhibitors fails and refractory GERC is considered (20).
Despite its advantage of higher sensitivity compared to T-SAP, C-SAP was only positive in less than half of patients with suspected GERC. Therefore, both C-SAP and T-SAP have limited diagnostic efficiencies and are unable to meet the needs for GERC identification. However, the diagnostic yield can be greatly improved when C-SAP is used in combination with AET or the number of reflux episodes over 24 h, as shown in our previous study (6).
There were several limitations of this study. First, SAP calculation depends on the symptoms reported by the patients. During MII-pH, the patients often forget to record the symptom events including cough and fail to label the symptom on time (29). One criticism is that SAPs may be inaccurate if the symptom reports are unreliable. MII-pH combined with synchronous cough frequency recording could greatly improve C-SAP accuracy (11). However, cough frequency recording is only used for study purpose at present and has not been employed in clinical practice. Second, it is not possible to record the other reflux-related symptoms. The SAP calculation used in the study is still recommended in the Lyon Consensus (5). Third, the placebo effect of anti-reflux therapies cannot be excluded since the study was uncontrolled. However, the therapies are logically expected to be effective for GERC based on their benefits in the treatment of GERD (5) and previous observations (4,20). Furthermore, it may be suggested only the patients without pathological reflux should be recruited to evaluate the efficacy of C-SAP in the study as the patients with pathological reflux would undergo anti-reflux treatment anyway. However, the pathological reflux does not always guarantee the therapeutic success of anti-reflux treatment in patients with suspected GERC (30) and cannot replace SAP in the diagnosis of GERC (6). Thus, we included the patients with pathological reflux since the study purpose was to compare the diagnostic yield of C-SAP and T-SAP for GERC and the resolution of cough but not the other reflux-related symptoms was designed as the primary outcome in response to anti-reflux therapy. Finally, cough resolution in response to anti-reflux therapy was set as the gold standard and may underdiagnose definitive GERC since some patients may not respond to treatment but still have GERC. It is therefore possible that C-SAP may be more valuable for GERC diagnosis than demonstrated when considering the outcome assessment used in the study.
Conclusions
C-SAP was superior to T-SAP for the identification of GERC, especially non-acid GERC in that it had higher sensitivity and a similar high specificity. C-SAP may therefore improve the diagnostic yield of GERC, even though its efficacy was suboptimal.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81670092), the Project of Science and Technology Commission of Shanghai Municipality (Nos. 20Y11902500, and 21140903400), and Clinical Research Project of Tongji Hospital of Tongji University (Nos. ITJ(ZD)2006 and TJ1801).
Footnote
Provenance and Peer Review: This article was a standard submission to the series “Cough Section” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1016/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1016/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1016/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1016/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tongji Hospital (No. LL(H)-2016-396). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. [Crossref] [PubMed]
- Kahrilas PJ. Chronic cough and gastroesophageal reflux disease: new twists to the riddle. Gastroenterology 2010;139:716-8. [Crossref] [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Kahrilas PJ, Altman KW, Chang AB, et al. Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. Chest 2016;150:1341-60. [Crossref] [PubMed]
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Li N, Chen Q, Wen S, et al. Diagnostic accuracy of multichannel intraluminal impedance-pH monitoring for gastroesophageal reflux-induced chronic cough. Chron Respir Dis 2021;18:14799731211006682. [Crossref] [PubMed]
- Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014;145:1264-70. [Crossref] [PubMed]
- Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994;107:1741-5. [Crossref] [PubMed]
- Ghillebert G, Janssens J, Vantrappen G, et al. Ambulatory 24 hour intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut 1990;31:738-44. [Crossref] [PubMed]
- Lai K, Shen H, Zhou XClinical Practice Guidelines for Diagnosis and Management of Cough-Chinese Thoracic Society (CTS) Asthma Consortium, et al. J Thorac Dis 2018;10:6314-51. [Crossref] [PubMed]
- Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010;139:754-62. [Crossref] [PubMed]
- Sifrim D, Dupont L, Blondeau K, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449-54. [Crossref] [PubMed]
- Bogte A, Bredenoord AJ, Smout AJ. Diagnostic yield of oesophageal pH monitoring in patients with chronic unexplained cough. Scand J Gastroenterol 2008;43:13-9. [Crossref] [PubMed]
- Wunderlich AW, Murray JA. Temporal correlation between chronic cough and gastroesophageal reflux disease. Dig Dis Sci 2003;48:1050-6. [Crossref] [PubMed]
- Qiu Z, Yu L, Xu S, et al. Cough reflex sensitivity and airway inflammation in patients with chronic cough due to non-acid gastro-oesophageal reflux. Respirology 2011;16:645-52. [Crossref] [PubMed]
- Sifrim D, Castell D, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004;53:1024-31. [Crossref] [PubMed]
- Blondeau K, Dupont LJ, Mertens V, et al. Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther 2007;25:723-32. [Crossref] [PubMed]
- Zhao T, Qiu Z, Wang L, et al. Validation of the reliability and clinical value of the simplified cough score. Zhonghua Quan Ke Yi Shi Za Zhi 2012;11:273-6.
- Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 1994;7:1246-53. [Crossref] [PubMed]
- Dong R, Xu X, Yu L, et al. Randomised clinical trial: gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough. Aliment Pharmacol Ther 2019;49:714-22. [Crossref] [PubMed]
- Asthma Group Branch of Respiratory Disease Chinese Medical Association. Guideline for the Diagnosis and Treatment of Cough (2015). Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39:323-54.
- Xu X, Yang Z, Chen Q, et al. Comparison of clinical characteristics of chronic cough due to non-acid and acid gastroesophageal reflux. Clin Respir J 2015;9:196-202. [Crossref] [PubMed]
- Herregods TVK, Pauwels A, Jafari J, et al. Ambulatory pH-impedance-pressure monitoring as a diagnostic tool for the reflux-cough syndrome. Dis Esophagus 2018;31:1-7. [Crossref] [PubMed]
- Irwin RS, Zawacki JK, Wilson MM, et al. Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near-total elimination of esophageal acid. Chest 2002;121:1132-40. [Crossref] [PubMed]
- Dickman R, Boaz M, Aizic S, et al. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 2011;17:387-94. [Crossref] [PubMed]
- Lv HJ, Qiu ZM. Refractory chronic cough due to gastroesophageal reflux: Definition, mechanism and management. World J Methodol 2015;5:149-56. [Crossref] [PubMed]
- Taghavi SA, Ghasedi M, Saberi-Firoozi M, et al. Symptom association probability and symptom sensitivity index: preferable but still suboptimal predictors of response to high dose omeprazole. Gut 2005;54:1067-71. [Crossref] [PubMed]
- Aanen MC, Weusten BL, Numans ME, et al. Effect of proton-pump inhibitor treatment on symptoms and quality of life in GERD patients depends on the symptom-reflux association. J Clin Gastroenterol 2008;42:441-7. [Crossref] [PubMed]
- Kavitt RT, Higginbotham T, Slaughter JC, et al. Symptom reports are not reliable during ambulatory reflux monitoring. Am J Gastroenterol 2012;107:1826-32. [Crossref] [PubMed]
- Kahrilas PJ, Howden CW, Hughes N, et al. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest 2013;143:605-12. [Crossref] [PubMed]


