
Trend of asthma prevalence and risk factors in the 20s age band in South Korea
Highlight box
Key findings
• In Korea, asthma prevalence was significantly increased in 20s age band from 2007 to 2018.
What is known and what is new?
• Data on the prevalence of asthma varies according to age, sex, and race. Asthma was known to be related to sex, smoking status, allergic disease, and BMI;
• The prevalence of asthma was increased only in their 20s significantly in South Korea, which might be related to the increase in the cases of allergic rhinitis and atopic dermatitis.
What is the implication, and what should change now?
• Considering health burden of asthma, further research is needed on the modifiable and treatable factors that increased the prevalence of asthma among subjects in their 20s.
Introduction
More than 339 million people worldwide had asthma in 2016, and the prevalence of asthma is increasing globally (1). To et al. (2) indicated that the global prevalence of physician-diagnosed asthma in adults was estimated to be 4.3% [95% confidence interval (CI): 4.2–4.4]. According to the European Community Respiratory Health Survey, a multicenter survey of adults aged 20 to 44 years in 48 centers, the asthma prevalence ranged from 2% to 3.3% at centers in Europe and from 8% to 11.9% at centers in the United Kingdom, New Zealand, and Australia (3). In Asian countries, including South Korea, the prevalence of asthma ranged from 0.7% to 11.9% (4). One study reported that the prevalence of asthma in South Korea increased from 1.55% in 2002 to 2.21% in 2015 (5).
Several risk factors of asthma have been identified, including smoking (6), allergens (7), respiratory infection (8), and obesity (9). In child, multiple gene susceptibility such as ORMDL3 and GSDML genes contributed to asthma (10), maternal diet and tobacco smoking were risk factors of asthma (11). Whereas, adult asthma is more frequent in obese and overweight women (12,13). Current and ex-smokers are known to be at increased risk of asthma and exacerbations. The total smoking amount (total pack-years) was also proportional to the asthma symptoms experienced, such as wheezing (14). Furthermore, a prospective study suggested that upper and lower respiratory tract infections were associated with the development of asthma in young adults (15).
Many studies have analyzed the prevalence and risk factors of asthma. However, the prevalence of asthma according to age is not well known. Thus, we analyzed the prevalence and the trend of prevalence of asthma according to age and analyzed the related risk factors. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-893/rc).
Methods
Study design
This study was conducted using open public data from the Korean National Health and Nutrition Examination Survey (KNHANES), a national surveillance system that has been assessing the health and nutritional status of Koreans since 1998 (16). The target recruitment group for the National Health and Nutrition Survey is the people living in Korea. The target of the survey is selected using a two-stage stratified colony sampling method in which the survey tools and households in the sampling frame for each year are the first and second extraction units, respectively (16). Stratified, multistage, clustered sampling based on national census data was used to ensure that the survey results represented the general Korean population (16). We analyzed the KNHANES data from 2007 to 2018 by merging the data from 2007–2009, 2010–2012, 2013–2015, and 2016–2018.
Subjects and data analysis
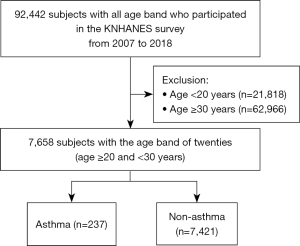
The number of subjects was 92,442, who were aged 1–80 years old. The KNHANES survey was conducted on a household bases and was analyzed based on the health survey questionnaire of the selected household. According to age bands, the subjects were classified with 10-year age band intervals to evaluate the increase in asthma prevalence (Figure 1). We also analyzed a subgroup of subjects with a specific age band in which asthma prevalence increased from 2007 to 2018. In addition, we used birth cohort analysis to determine whether there was the casual relationship between the birth period and asthma.
Definition of asthma and covariates
The self-reported questionnaire provided information on the subjects’ asthma condition, household income, education, allergic rhinitis, atopic dermatitis, smoking status, environmental tobacco smoking, and body mass index (BMI).
These factors were simplified by newly classifying them into two groups because each group was too small to be further subdivided. In original survey, household income levels were divided into four categories as followed: ‘lower’, ‘middle-lower’, ‘middle-upper’, and ‘upper’ quartiles. We classified household income into two categories for statistical analysis. ‘Lower’ and ‘middle-lower’ quartiles were classified into ‘lower-half’, and ‘middle-upper’, and ‘upper’ quartiles were classified into ‘higher-half’. The questionnaire on the level of education was divided into ‘village schools’, ‘no education’, ‘elementary schools’, ‘middle schools’, ‘high schools’, ‘two or three years of college’, ‘four-year universities’, and ‘graduate schools’, we classified the education level as below high school and above two or three years of college.
Asthma was defined when the study subjects responded positively to the following question: “Have you been diagnosed with asthma by your doctors?” Allergic rhinitis and atopic dermatitis were determined when the study subjects responded positively to the following question: “Have you been diagnosed with the disease by your doctors?” Smokers were determined by those who replied with “more than 100 cigarettes” to the following question: “Have you ever smoked in your life?” Nonsmokers were determined by those who replied to the same question with “never” or “less than 100 cigarettes”. Furthermore, we defined ‘current smokers’ by those who replied with “every day” or “occasionally” to the following question: “Are you currently smoking?”. ‘Ex-smokers’ were determined by those who replied to the same question with “not at present”. We defined as ‘ever smokers’ for both ‘current smokers’ and ‘ex-smokers’. Environmental tobacco smoking is defined as the exposure to secondhand smoking in the home. BMI was calculated as a subject’s weight in kilograms divided by the square of their height in meters. The BMIs were classified according to the criteria of the Korean Society for the Study of Obesity; thus, a BMI ≥25.0 kg/m2 was defined as obesity (17).
Statistics analysis
Statistical analyses were conducted using SPSS version 24.0 (IBM Company). Survey sample weights were used in all analyses to produce estimates representing the noninstitutionalized civilian Korean population. To identify changes in prevalence rate trends, joinpoint regression was estimated for every age group. By using prevalence rates as inputs, this method identifies the year when a trend of asthma prevalence is produced, it calculates the annual percentage change (APC) in rates between trend-change points, and it also estimates the average annual percentage change (AAPC) in the whole study period. A P<0.05 was considered statistically significant. Multiple logistic regression analyses with a complex sample design were conducted to determine the risk factors for asthma. Each category was marked with different numbers because of missing values such as “non-responses” and “unknown;” thus, the missing values were treated as valid values and reflected in the statistical analysis by following the KNHANES guidelines.
Study approval and informed consent
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Bioethics Committee of Asan Medical Center, Ulsan National University (IRB No. 20200849), which waived the requirement for informed consent because of the retrospective nature of the study.
Results
Asthma prevalence according to age band from 2007 to 2018 in South Korea
From 2007 to 2018, the prevalence of asthma was 4.5%. Furthermore, the prevalence of asthma according to age band decreased gradually, with the lowest level of 2.1% in the 40s age band. However, since then, the prevalence of asthma has gradually increased and reached 6.1% in individuals who are 70 years and older (Figure S1).
From 2007 to 2018, only the 20s age band showed a statistically significant increase in the prevalence of asthma (Figure 2). The asthma prevalence in the 20s age band was 0.7% in 2007 and 5.1% in 2018 (P<0.001 for joinpoint regression).
Risk factors related to asthma in the 20s age band
There were 7,658 subjects in the 20s age band, with 237 and 7,421 subjects having and not having asthma, respectively. Among the 7,658 subjects in their 20s, the average age was similar to that of the asthma group (24.1 years) and that of the non-asthma group (24.7 years). The proportion of men was higher in the asthma group (54.9% in the asthma group and 43.9% in the non-asthma group, P=0.018), and there were more ever-smokers in the asthma group than in the non-asthma group (43.9% in the asthma group and 37.5% in the non-asthma group, P=0.009). The proportions of allergic rhinitis (44.6% in the asthma group and 20.6% in the non-asthma group, P<0.001) and atopic dermatitis (25.3% in the asthma group and 8.0% in the non-asthma group, P<0.001) were higher in the asthma group than in the non-asthma group. The tendency of other variables such as household income, education status, environmental tobacco smoking, and obesity was similar to those of the 20s age band regardless of asthma (Table 1).
Table 1
| Characteristics | Asthma | Non-asthma | P value |
|---|---|---|---|
| Number of subjects | 237 | 7,421 | – |
| Mean age, years | 24.1 | 24.7 | – |
| Sex (male)* | 130/237 (54.9%) | 3,193/7,421 (43.9%) | 0.018 |
| Lower-household income* | 79/236 (33.5%) | 2,516/7,343 (34.3%) | 0.915 |
| Education (Junior college or higher)* | 194/237 (81.9%) | 6,138/7,414 (82.8%) | 0.905 |
| Ever-smokers* | 104/237 (43.9%) | 2,779/7,414 (37.5%) | 0.009 |
| Environmental tobacco smoking* | 197/237 (83.1%) | 6,281/7,410 (84.8%) | 0.254 |
| Allergic rhinitis* | 82/184 (44.6%) | 1,150/5,584 (20.6%) | <0.001 |
| Atopic dermatitis* | 60/237 (25.3%) | 590/7,421 (8.0%) | <0.001 |
| Obesity* | 67/230 (29.1%) | 1,657/7,343 (22.6%) | 0.051 |
*, target subjects over the total number of subjects with percent in parentheses. Lower household income: bottom two quartiles of income divided by quartiles; ever-smokers: smoked more than 100 cigarettes in the past or now smoking daily or occasionally; obesity: BMI ≥25.0 kg/m2 (BMI = weight in kilograms/square of height in meters). BMI, body mass index.
In a univariate logistic regression of the subjects in the 20s age band, asthma prevalence was related to male sex [odds ratio (OR), 1.51; 95% CI: 1.15–1.99], allergic rhinitis (OR, 3.14; 95% CI: 2.34–4.21), atopic dermatitis (OR, 4.44; 95% CI: 3.23–6.10), and ever-smoking (OR, 1.42; 95% CI: 1.09–1.86). Furthermore, in subjects in the 20s age band, multivariate logistic regression analysis revealed that asthma was related to allergic rhinitis (OR, 2.78; 95% CI: 2.03–3.81) and atopic dermatitis (OR, 4.13; 95% CI: 2.85–5.98). However, asthma was not related to male sex (OR, 1.43; 95% CI: 1.00–2.04), higher income (OR, 1.03; 95% CI: 0.75–11.40), higher education (OR, 0.98; 95% CI: 0.64–1.50), ever-smoking (OR, 1.32; 95% CI: 0.93–1.88), environmental tobacco smoking (OR, 1.23; 95% CI: 0.78–1.94), and obesity (OR, 0.99; 95% CI: 0.70–1.38) (Table 2).
Table 2
| Potential risk factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | P value | Adjusted OR | 95% CI | P value | ||
| Sex (male) | 1.51 | 1.15–1.99 | 0.003 | 1.43 | 1.00–2.04 | 0.05 | |
| Higher income | 1.02 | 0.76–11.40 | 0.92 | 1.03 | 0.75–1.40 | 0.87 | |
| Higher education | 1.04 | 0.72–1.48 | 0.85 | 0.98 | 0.64–1.50 | 0.94 | |
| Allergic rhinitis | 3.14 | 2.34–4.21 | <0.001 | 2.78 | 2.03–3.81 | <0.001 | |
| Atopic dermatitis | 4.44 | 3.23–6.10 | <0.001 | 4.13 | 2.85–5.98 | <0.001 | |
| Ever-smokers | 1.42 | 1.09–1.86 | 0.01 | 1.32 | 0.93–1.88 | 0.12 | |
| Environmental tobacco smoking | 0.83 | 0.58–1.19 | 0.32 | 1.23 | 0.78–1.94 | 0.37 | |
| Obesity | 1.34 | 1.00–1.79 | 0.05 | 0.99 | 0.70–1.38 | 0.93 | |
This table covers 5,641 subjects with information on allergic diseases (allergic rhinitis and atopic dermatitis). Higher income: top two quartiles of income divided by quartiles; higher education: above two or three years of college; ever-smokers: smoked more than 100 cigarettes in the past or now smoking daily or occasionally; obesity: BMI ≥25.0 kg/m2 (BMI = weight in kilograms/square of height in meters). BMI, body mass index; CI, confidence interval; OR, odds ratio.
The prevalence rates of allergic rhinitis and atopic dermatitis in the 20s age band increased from 2007 to 2018. The prevalence of allergic rhinitis was 17.2% in 2007 and 23.5% in 2018 (P<0.001 via the chi-square test; Figure S2), and the prevalence of atopic dermatitis was 5.9% in 2007 and 11.7% in 2018 (P=0.001 via the chi-square test; Figure S3). These results tend to be similar to the increase in the prevalence of asthma in the 20s age band.
Discussion
This study identified that the prevalence of asthma in the 20s age band significantly increased in South Korea from 2007 to 2018. Furthermore, increased of asthma prevalence was related to allergic rhinitis and atopic dermatitis.
This is the first study that analyzed the trend of asthma prevalence in South Korea by age group. Asthma is a chronic disease that accounts for 1 in every 250 deaths worldwide, with 12 million people experiencing acute exacerbation every year in the United States (18).
The modifiable and treatable factors for the increase in the prevalence of asthma in the 20s age band were not clearly identified in this study. However, considering the economic and health burden of asthma (19), it is important to monitor prevalence, also, it is necessary to divide and manage by age band. Considering the need for long-term care, the increase in the prevalence of asthma in the 20s age band in Korea is an important discovery. This study emphasized the importance of people in charge of healthcare prevention recognizing and managing the prevalence of asthma according to age bands.
Studies conducted on the basis of the National Health Insurance Sharing Service reported an increase in the prevalence of asthma in South Korea (1.55% in 2002 and 2.21% in 2015), and the prevalence of asthma in the 20s age band seemed to be steadily increasing (5). This is consistent with the results of the current study.
In European countries, the weighted prevalence of diagnosed asthma was 6.2% in 2018 (20). Higher prevalence of asthma in Western countries might be due to higher rates of obesity (16). Additionally, ‘hygiene hypothesis’ that frequent bacterial infection in childhood could lead to adverse association with allergic disease might be related to the high prevalence of asthma in the Western countries (21). The trend of global prevalence varies significantly from country to country owing to the absence of large-scale standardized data; however, there were no signs of declining prevalence, and prevalence is still increasing in many parts of the world (22).
Data on asthma prevalence trends by age group are limited; however, the prevalence of asthma in Sweden has been on the rise for 30 years and has been increasing in all age groups, except in men aged 65–74 years old (23). US data during the last 20 years show an increase in asthma prevalence in older people aged 65 years or older (24). In the current study, asthma prevalence increased in people in their 20s but not in other age groups. Especially, asthma diagnosis was reduced in the 70 and older age group in our database. In a recently published paper (25), supporting evidences for declining asthma prevalence in elderly was analyzed. In Korea, there were issues related to exposure to humidifier disinfectants until 2011, which might have contributed to increasing the prevalence of asthma in old age who are more fragile. In addition, because of the influenza and Mycoplasma pandemic around 2010, the prevalence of asthma in the elderly might have increased at that time, and then gradually have decreased. We considered that the pandemic and the pollution of certain periods have temporarily increased the prevalence of asthma in older age who might be more vulnerable.
We performed a birth cohort analysis to confirm whether an increase in asthma prevalence in the 20s age band was the result of bias among those born at a particular time. Thus, the prevalence of asthma was analyzed annually among those in the 20s age band at the beginning of the study (i.e., in 2007), but there was no significant increase in the trend of asthma prevalence (Figure S4). The increase in asthma prevalence in the 20s age was a true finding, not related to birth bias at a particular time.
Univariate and multivariate analyses were conducted to determine the factors affecting the increase in asthma prevalence in people in their 20s. Univariate analysis showed that asthma prevalence has a significant relationship with male sex, allergic rhinitis, atopic dermatitis, and ever-smoking. Furthermore, multivariate analysis showed that asthma prevalence has a significant relationship with allergic rhinitis and atopic dermatitis. In addition, young adults might be treated earlier due to their high awareness, but in this study, it was limited because there was no database until time to treatment. Besides, Western lifestyles such as dietary factors might have been more reflected in the young adult group, which could have contributed to the increase of asthma (19).
According to previous studies, asthma-related hospital visits and hospitalization were higher in boys under 14 years old (16). However, after puberty, asthma is more common in women and is more severe (16). This is attributed to the differences in anatomical structures between men and women: the ratio of forced flow rate to forced vital capacity is higher in women than in men (17). Furthermore, female hormones in relation to menstruation can also have an effect on asthma prevalence (12). However, in the current study, men had a higher prevalence of asthma than women in the 20s age band. Considering that the smoking rate among men in their 20s was higher (proportion of ever-smoking was 61.0% in the men and 20.8% in the women in the 20s band on our database), we believe that the association between smoking status and asthma may be affected (Figure S5).
Studies showed that the risk of developing asthma was significantly higher in smokers and that the risk of asthma increased even when exposed to environmental tobacco smoking (26). In adults, smokers have a higher risk of asthma than never-smokers, and this effect is more pronounced in women than in men (6). Smoking increased bronchial hypersensitivity, eosinophilia, and helper T-cell cytokine response after exposure to allergens (27). Additionally, continuous exposure to smoking can promote airway remodeling and produce asthma by activating epithelial cells (28).
Many studies have revealed the similarities of the pathophysiology of asthma and the relation between asthma and allergic disease (29,30). We found a relationship between the increased prevalence of asthma in the 20s age band and allergic diseases such as allergic rhinitis and atopic dermatitis. There was some phenotype of asthma, defined as visible traits that were the outcome of a combination of inherited and environmental factors (31). Allergic asthma, the most common phenotype (32), occurs more often in people under 30 years old, and non-allergic asthma is more common in late adulthood (33). With the increase in various indoor and outdoor environmental factors (i.e., fine dust, air pollution, influenza virus infection) (34) and development with Westernized lifestyles (35), the prevalence of allergic disease increases especially in early adulthood which was less exposed to irritating factors such as occupational exposure and smoking and vulnerable, we suggest that was associated with increased prevalence of asthma in the age 20s (25,35).
A study had shown that the prevalence of atopic dermatitis in preschool children has decreased significantly over the past 10 years, and there had been no significant increase in the prevalence of allergic rhinitis in Korea (25). In our data, trend of asthma prevalence at the age of 0 to 9 was a significant decreased (6.5% in 2007, 2.2% in 2018; P value <0.001 for joinpoint regression). We posit that the increase in allergic disease affected the increase in asthma prevalence in the age 20s.
There are several limitations to this study. First, this study was conducted on samples representing South Korea; therefore, the results may be difficult to generalize in other racial groups or countries. Second, this study was a cross-sectional design; therefore, it was difficult to ascertain a causal relationship for asthma development. Third, asthma defined as the physician-diagnosed asthma in this study, it could be overestimation or underestimation compared to asthma diagnosed based on laboratory test results. Underestimation of asthma was associated with under-reporting of symptoms by patients. In opposite, patients with physician-diagnosed with asthma indicate that 30–35% of subjects actually do not have current asthma, it associated with overestimation (36). Further research is required on the prevalence of asthma diagnosed based on laboratory test in the future. Fourth, the classification of asthma according to phenotype is important, however in this study, test results such as immunoglobulin E (IgE) and skin prick test were limited. We could not conduct the analysis of asthma phenotype, so further research would be needed.
This study was based on representative data in Koreans and was conducted on young subjects in the 20s age band. Therefore, it is meaningful that the research was conducted only on asthma by minimizing the possibility of mistaking asthma for chronic obstructive pulmonary disease (COPD) or asthma-COPD combination.
Conclusions
In conclusion, from 2007 to 2018, the prevalence of asthma significantly increased in the 20s age band in South Korea. This finding may be related to the increase in allergic rhinitis and atopic dermatitis. Further research is needed to discover the causes of increased asthma prevalence in subjects in their 20s.
Acknowledgments
Editorial support in the form of writing assistance, collation of author’s comments, and grammatical editing were provided by the Scientific Publications Team of Asan Medical Center. Additionally, Asan Medical Library provided support in the preparation of the tables, figures, and references.
Funding: This study was supported by the University of Ulsan College of Medicine.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-893/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-893/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-893/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Bioethics Committee of Asan Medical Center, Ulsan National University (IRB No. 20200849), which waived the requirement for informed consent because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. [Crossref] [PubMed]
- To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012;12:204. [Crossref] [PubMed]
- Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1996;9:687-95. [Crossref] [PubMed]
- Song WJ, Kang MG, Chang YS, et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy 2014;4:75-85. [Crossref] [PubMed]
- Lee E, Kim A, Ye YM, et al. Increasing Prevalence and Mortality of Asthma With Age in Korea, 2002-2015: A Nationwide, Population-Based Study. Allergy Asthma Immunol Res 2020;12:467-84. [Crossref] [PubMed]
- Piipari R, Jaakkola JJ, Jaakkola N, et al. Smoking and asthma in adults. Eur Respir J 2004;24:734-9. [Crossref] [PubMed]
- Polosa R, Al-Delaimy WK, Russo C, et al. Greater risk of incident asthma cases in adults with allergic rhinitis and effect of allergen immunotherapy: a retrospective cohort study. Respir Res 2005;6:153. [Crossref] [PubMed]
- Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010;376:826-34. [Crossref] [PubMed]
- Barnthouse M, Jones BL. The Impact of Environmental Chronic and Toxic Stress on Asthma. Clin Rev Allergy Immunol 2019;57:427-38. [Crossref] [PubMed]
- Meyers DA, Bleecker ER, Holloway JW, et al. Asthma genetics and personalised medicine. Lancet Respir Med 2014;2:405-15. [Crossref] [PubMed]
- Trivedi M, Denton E. Asthma in Children and Adults-What Are the Differences and What Can They Tell us About Asthma? Front Pediatr 2019;7:256. [Crossref] [PubMed]
- Shah R, Newcomb DC. Sex Bias in Asthma Prevalence and Pathogenesis. Front Immunol 2018;9:2997. [Crossref] [PubMed]
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018;141:1169-79. [Crossref] [PubMed]
- Kim SY, Sim S, Choi HG. Active and passive smoking impacts on asthma with quantitative and temporal relations: A Korean Community Health Survey. Sci Rep 2018;8:8614. [Crossref] [PubMed]
- Rantala AK, Jaakkola MS, Mäkikyrö EM, et al. Early Respiratory Infections and the Development of Asthma in the First 27 Years of Life. Am J Epidemiol 2015;182:615-23. [Crossref] [PubMed]
- Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69-77. [Crossref] [PubMed]
- Seo MH, Lee WY, Kim SS, et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J Obes Metab Syndr 2019;28:40-5. [Crossref] [PubMed]
- Fergeson JE, Patel SS, Lockey RF. Acute asthma, prognosis, and treatment. J Allergy Clin Immunol 2017;139:438-47. [Crossref] [PubMed]
- Alwarith J, Kahleova H, Crosby L, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev 2020;78:928-38. [Crossref] [PubMed]
- Khan A, Sternbach N, Kamat S, et al. Prevalence of asthma in France, Germany, Italy, Spain, and the United Kingdom, based on the 2018 European National Health and Wellness Survey. Chest 2020;158:27A. [Crossref]
- Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol 2013;13:70-7. [Crossref] [PubMed]
- Sears MR. Trends in the prevalence of asthma. Chest 2014;145:219-25. [Crossref] [PubMed]
- Pelkonen MK, Notkola IK, Laatikainen TK, et al. 30-year trends in asthma and the trends in relation to hospitalization and mortality. Respir Med 2018;142:29-35. [Crossref] [PubMed]
- Elflein J. Percentage of current asthma in the United States 2001-2018, by age group. Statista. 2020. Available online: https://www.statista.com/statistics/251963/percentage-of-current-asthma-in-the-us-by-age-group-since-2001/ (accessed Oct 13 2021).
- Ha J, Lee SW, Yon DK. Ten-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008-2017. Clin Exp Pediatr 2020;63:278-83. [Crossref] [PubMed]
- Jayes L, Haslam PL, Gratziou CG, et al. SmokeHaz: Systematic Reviews and Meta-analyses of the Effects of Smoking on Respiratory Health. Chest 2016;150:164-79. [Crossref] [PubMed]
- Seymour BW, Schelegle ES, Pinkerton KE, et al. Second-hand smoke increases bronchial hyperreactivity and eosinophilia in a murine model of allergic aspergillosis. Clin Dev Immunol 2003;10:35-42. [Crossref] [PubMed]
- Lee JS. The association with smoking and asthma. Allergy Asthma Respir Dis 2018;6:137-40. [Crossref]
- Guibas GV, Mathioudakis AG, Tsoumani M, et al. Relationship of Allergy with Asthma: There Are More Than the Allergy "Eggs" in the Asthma "Basket". Front Pediatr 2017;5:92. [Crossref] [PubMed]
- Bergeron C, Hamid Q. Relationship between Asthma and Rhinitis: Epidemiologic, Pathophysiologic, and Therapeutic Aspects. Allergy Asthma Clin Immunol 2005;1:81-7. [Crossref] [PubMed]
- Kuruvilla ME, Lee FE, Lee GB. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol 2019;56:219-33. [Crossref] [PubMed]
- Akar-Ghibril N, Casale T, Custovic A, et al. Allergic Endotypes and Phenotypes of Asthma. J Allergy Clin Immunol Pract 2020;8:429-40. [Crossref] [PubMed]
- Pakkasela J, Ilmarinen P, Honkamäki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med 2020;20:9. [Crossref] [PubMed]
- Lee SW, Yon DK, James CC, et al. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: Age-stratified time-series analysis. J Allergy Clin Immunol 2019;144:1542-1550.e1. [Crossref] [PubMed]
- Zhang Y, Zhang L. Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol Res 2019;11:156-69. [Crossref] [PubMed]
- Aaron SD, Boulet LP, Reddel HK, et al. Underdiagnosis and Overdiagnosis of Asthma. Am J Respir Crit Care Med 2018;198:1012-20. [Crossref] [PubMed]



