Vascular access for extracorporeal life support: tips and tricks
Introduction
The contemporary extracorporeal life support (ECLS) techniques are the results of more than a century of technical innovations, scientific knowledges, and clinical practices. The proof of concept of both the ECLS and the extracorporeal hematosis has been established through different positive clinical experiences in adults and neonatal cohorts (1-4). The concept of ECLS is the association of: (I) a short to mid term mechanical extracorporeal support; (II) the extracorporeal clearance of carbon dioxide (CO2); (III) the extracorporeal oxygenation according the ECLS flow; and (IV)—depending the configuration of the circuit—the extracorporeal cardiocirculatory support. The aim of the ECLS is to supply the failing respiratory and/or cardiocirculatory system(s) until: the recovery (bridge to recovery), the transplantation (bridge to transplantation), the change to another ECLS device/configuration (bridge to bridge), or the change of cares strategy (bridge to decision).
“ECLS” is a generic term including all the extracorporeal mechanical support techniques. In thoracic surgery, the ECLS techniques mostly used are: (I) the extracorporeal membrane oxygenation (ECMO); (II) the extracorporeal CO2 removal (ECCO2R); and (III) the pumpless device “Novalung” (Novalung GmbH, Hechingen, Germany). Nevertheless the clinical practice discriminates the term “ECLS” than the term “ECMO” with a cardiorespiratory support connotation and an exclusive respiratory support meaning, respectively. In this terminological context, the vascular access of the ECLS is venoarterial (VA) and those of the ECMO are VA, venovenous (VV), or arteriovenous (AV). In this review we will use the term “ECLS” as a generic term for the overall extracorporeal mechanical support techniques available. The objective of this review is to describe both the rational and the techniques of the different ECLS techniques used in thoracic surgery and lung transplantation (LTx). We specifically will focus on the vascular approaches.
Rational of the techniques of ECLS
Basically, the ECLS circuit is a minimized cardiopulmonary bypass (CPB) circuit including (I) an outflow cannula prolonged by (II) an outflow line connected with (III) a non-occlusive centrifugal pump. The pump injects the blood through (IV) a membrane oxygenator in (V) the inflow line. The arterial blood is restituted to the patient through (VI) an inflow cannula.
In a terminological point of view the drainage site is positioned prior the injection site. As example the term “VA ECMO” means an ECLS using the venous and the arterial systems for drainage and injection, respectively. A “femoro-right internal jugular VV ECMO” corresponds to a VV ECMO (excepted the Novalung, all the ECLS techniques used the venous system for drainage) having a femoral cannula for drainage and the right internal jugular vein as injection site.
The ECLS techniques and their properties are defined by both the specificities of the circuit and the cannulation sites.
The Figure 1 illustrates the circuit of a left femoral-right internal jugular VV ECMO.
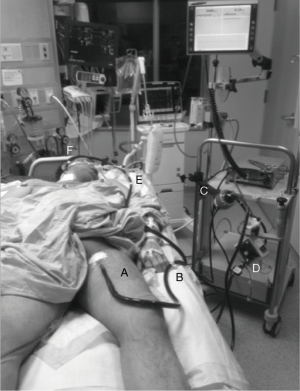
The Table 1 enumerates the different ECLS techniques used in thoracic surgery and their clinical properties.

Full table
The extracorporeal membrane oxygenation (ECMO)
The venovenous extracorporeal membrane oxygenation (VV ECMO)
VV ECMO is the most frequently ECLS technique used in thoracic surgery. VV ECMO represents the most efficient ECLS technique for extracorporeal respiratory support. In this technique, the artificial lung is in series with the failing native lungs. Thus, the oxygenated and decarboxylated blood goes through the native lungs decreasing the pulmonary vascular resistances and allowing an optimal oxygenation of the lungs, the heart, and the brain. VV ECMO allows to perform a protective mechanical ventilation strategy (5-8).
The cornerstone of the VV ECMO is to dissociate the decarboxylation and the oxygenation functions. The CO2 removal function is dependent on the flow of the gas mixture through the membrane. This gas mixture flow is called sweep gas flow. More elevated is the sweep gas flow, higher is the clearance of the CO2. The CO2 removal does not depend on the flow of blood in the ECMO circuit. Conversely, the oxygenation is proportional to the ECMO flow. The oxygenation also depends on the delivered fraction of oxygen (FdO2) in the gas mixture. The level of the sweep gas flow does not influence the oxygenation (7,8).
Hence, VV ECMO is indicated to patients with severe, isolated, and refractory respiratory failures. An associated heart failure due to hypoxemia and/or respiratory acidosis may also be successfully treated by VV ECMO.
On VV ECMO, the main limiting factor is the oxygenation. To prevent this issue, oxygen requirements should be assessed and estimated prior to the ECMO insertion and largest cannulas should be used to decrease the circuit resistances.
The venoarterial extracorporeal membrane oxygenation (VA ECMO)
VA ECMO is performed for cardiocirculatory support with or without respiratory failure. In this ECLS technique, the artificial lung is in parallel with the native lungs. In peripheral configuration, the blood perfusing the brain and the heart results from the mixing of the blood coming from the native lungs and from the ECMO. Depending the left ventricular function, the blood destined to the vital organs (brain, heart, lungs) and the upper hemicorpus may contain a low PaO2 (7,8). Hence peripheral VA ECMO should not be used as a first-line ECLS strategy in case of isolated lung failure.
Central VA ECMO is used as a conventional CPB for respiratory and circulatory intraoperative support. Comparing CPB, central VA ECMO needs a lower level of heparin and induces less inflammation. Nevertheless central VA ECMO has the main disadvantages to have no vent and sucker and to not allow cardioplegia.
In a haemodynamic point of view, VA ECMO provides a cardiocirculatory support decreasing the right ventricle preload and increasing the left ventricle afterload. The level of the cardiocirculatory support is determined by the ECMO flow. Thus, the ECMO flow may be the limiting factor on this technic. The injection cannula should be large enough to obtain an ECMO flow higher than the patient cardiac output [targeted maximum ECMO flow = calculated ideal cardiac output + 1.5 − 2.0 liters per minute (LPM); to compensate the O2 consumption related to the catabolism].
The extracorporeal CO2 removal (ECCO2R)
ECCO2R devices are used to perform a partial clearance of the CO2. They are indicated in isolated hypercapnia. The rational of this ECLS technic is based on the clinical improvement of patient suffering of respiratory acidosis by decreasing their levels of CO2 production by 33% (9). Thus, the first indication was the hypercapnic failure of chronic obstructive pulmonary disease patients. Currently the indications are expanded to isolated hypercapnia. The manufactured circuits—Hemolung® Respiratory Assist System (ALung Technologies, Pittsburgh, PA, USA), ilA activve® (Novalung, Hechingen, Germany), and Hemodec DECAPsmart® (Hemodec, Salerno, Italy)—include a small (from 14 to 24 Fr) venous dual lumen catheter. Because of the circuit resistances, the flow of the circuit (<1 LPM) does not allow the oxygenation function. The level of the decarboxylation is determined by the sweep gas flow through the membrane and may reach 90–100 mLPM. These respiratory dialysis techniques are used with the same levels of heparin than conventional VV ECMO. Hemorrhagic issues are not decreased with this ECLS technique (9).
The Novalung (Novalung GmbH, Hechingen, Germany)
Novalung is a pumpless technique of ECLS. The circuit is simply composed by: an outflow cannula, an outflow line, a low resistance membrane oxygenator, an inflow line, and an inflow cannula.
The arteriovenous (AV) Novalung
Initially, the Novalung was used as an AV ECMO draining the blood from the femoral artery and injecting it in the femoral vein. In this configuration, the blood flow was entirely performed by the cardiac output. The flow reached—depending the cardiac function—2.5 LPM; allowing a complete CO2 clearance and a partial oxygenation.
With the occurrence of the less invasive ECCO2R technology (same clinical indications with none arterial cannulation/issue and easier ambulation), it seems that this AV Novalung indication is going to significantly decrease.
The pulmonary artery-left atrium Novalung
The pumpless pulmonary artery-left atrium Novalung (PA-LA Novalung) technique is used to bridge to LTx severe pulmonary hypertension patients having a refractory respiratory or cardiorespiratory failure. In this ECLS technique, the blood is drained from the PA and injected in the LA. The flow is mainly generated by the gradient of pressures between the PA and the LA. Both PA and LA cannulas should be large enough to not impair this gradient of pressures. The PA-LA Novalung has the properties of (I) decarboxylation; (II) oxygenation; and (III) right ventricle remodeling. Oxygenation is allowed by both the central configuration of the Novalung and the flow (1.0–3.0 LPM) through the circuit. The right ventricle remodeling is allowed by the low resistance of the membrane oxygenator decreasing the right ventricle afterload.
Cannulation: general points and anticoagulation strategies
The cannulation techniques and the ECLS efficiency will depend on both the cannulation sites and the venous or arterial nature of the cannulated vessel.
Central cannulation
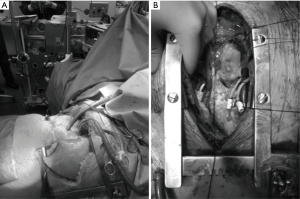
Central VA ECMO and PA-LA Novalung are inserted under general anesthesia using most of the time a Clamshell incision and a sternotomy incision, respectively. Central VA ECMO as intraoperative support is inserted as a conventional CPB through the thoracotomy incision with the aim to be weaned at the end of the procedure. Prolonged central VA ECMO or PA-LA Novalung cannulas should be inserted through two skin incisions placed below the inferior part of the thoracotomy incision. In central configurations, each cannulation sites must be secured with purse-string sutures and tourniquets tied around the cannula. On central VA ECMO, to prevent air embolization around the drainage cannula it may be helpful to tie an umbilical tape around the cannula and the atrial wall.
The Figure 2 illustrates the cannulation of a central VA ECMO and a PA-LA Novalung.
Peripheral cannulation
Peripheral ECLS may be inserted surgically or percutaneously. Usually venous sites are cannulated percutaneously whereas arterial cannulations are performed surgically. The rational of this paradigm is related to the ECMO removal. Hence once ECLS are removed, a single 10 minutes compression period—associated with a percutaneous suture—allows to obtain an efficient hemostasis of the venous cannulation sites. Rather, the cannulated artery should be fixed and the vessels dissections and controls may be challenging in case of previous percutaneous cannulation; particularly if this cannulation was complex with iterative punctures.
Concerning the ECLS insertion, it is common to realize the vessels percutaneous approach using an ultrasound guidance. This strategy allows to prevent vessel injuries and higher risks of bleeding. The percutaneous approach is performed with the Seldinger technique (puncture/insertion of a guide wire/progressive dilatation/and cannulation).
The surgical approach allows to safely introduce the cannula under vision control. After proximal and distal control of the vessels, a doubled purse-string suture around the insertion point will prevent air embolisms and hemorrhagic issues and also secure the positioning of the cannula. The cutaneous insertion site(s) of the cannula(s) should differ from the skin incision done for the vessel(s) approach. It allows to (I) easily insert the cannula(s) with a low angle (≤30°) and (II) avoid external bleeding around the cannula(s).
In all configurations and to avoid external bleeding, the cutaneous insertion point of the cannula should be closely tie around the cannula using separated stitches and/or a cutaneous purse string suture. After control of the correct positioning, the cannulas must be fixed to the skin in several points.
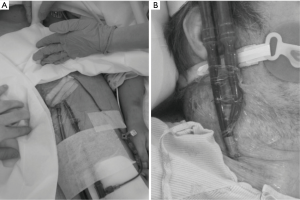
The Figure 3 illustrates the cannulation of a peripheral VA ECMO and a single-site VV ECMO.
Positioning of the cannulas
The cannulas must be correctly positioned for an optimal efficiency of the ECLS device. The Figure 4 shows a chest X-ray illustrating the correct positions of the cannulas of a double-site VV ECMO.
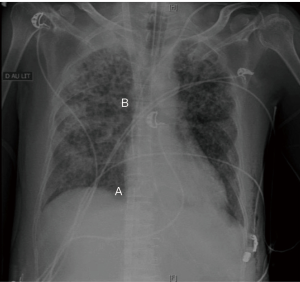
On VA or double site VV ECMO, the rule is to place the tip of the drainage cannula at the junction between the cannulated vena cava and the right atrium. A perfect positioning of the drainage cannula would eliminate a defect of the outflow due to the cannula position and would guide to a patient volume status issue or an abnormal increase of the ECLS resistances (clots, deficiency of the oxygenator, injection issue). The good positioning of the outflow cannula may be estimated clinically but should be confirmed by radiology (chest X-ray, transthoracic ultrasound, transesophageal ultrasound, per-procedure fluoroscopy guidance).
The positioning of the tip of the injection cannula is less challenging excepted on double-site VV ECMO. Indeed to obtain an optimal oxygenation of the vital organs, the blood coming from the ECMO should be injected as close as possible to the heart; ideally in the junction between the cannulated vena cava and the right atrium. On double site VV ECMO, if the two cannulas are to close the injected blood may be directly drained by the ECMO. This process is called “recirculation” and is also promoted by a high ECMO flow. In this issue, the ECMO works as a close circuit. Recirculation may be assessed clinically (flashs of arterial blood in the drainage line) and quantified biologically comparing blood gas values (pre- and post-membrane blood gas, patient central venous blood gas).
On VA ECMO if a cardiorespiratory support is needed, it is necessary to: (I) insert the injection cannula in the right axillary artery via a vascular prothesis or (II) to add an extra second venous injection cannula to perform a veno-arteriovenous ECMO.
On PA-LA Novalung, the tip of the drainage cannula should be placed in the pulmonary trunk whereas the one from the injection cannula is placed in the left atrium above the right superior pulmonary vein.
ECCO2R devices may be implanted in the internal jugular veins, subclavian veins, or femoral veins with similar results. Nevertheless to promote the patient mobility it is encouraged to keep the femoral veins free.
Vascular issues
The vascular issues are prevented by (I) a surgical or an ultrasound-guided percutaneous approach and (II) a smooth insertion of the cannulas. The nature of the vascular issues depends on the nature of the cannulated vessel.
In peripheral configuration, the main and most critical arterial issue is the acute limb ischemia. It is prevented by the use of a distal reperfusion cannula. A smaller injection cannula may also be used but it is to the detriment of both the ECLS flow and the level of the cardiocirculatory support. At the time of the peripheral VA ECMO removal, it may be helpful to perform an embolectomy of the distal trunks to remove clots and restitute a healthy distal vascular bed.
Arterial dissections or arterial wall injuries should be fixed surgically. Isolated vein injuries can be fixed by single compression. After ECMO removal, arterial stenosis or vein thrombosis are treated by usual manner.
Anticoagulation strategies
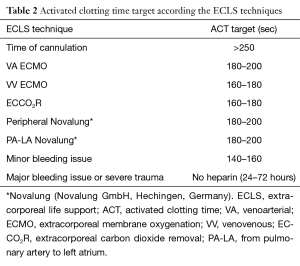
The anticoagulation strategy depends on the ECLS technique employed. At the time of cannulation a bolus of heparin is realized (from 3,000 to 5,000 UI of heparin depending the clinical status and the body weight of the patient). Implantation of the cannula may start once the activated clotting time is above 250 seconds for both central and peripheral cannulation. Protamine may be added at the end of the ECLS insertion in case of bleeding. Nevertheless if there is no hemorrhagic concern, it is better to not reverse the heparin.
In case of polytrauma or isolated severe chest trauma with major risk of bleeding, it is possible to use none heparin for a short delay (24–72 hours).
The Table 2 enumerates the activated clotting time target according the ECLS techniques.
Full table
Conventional configurations
VV ECMO and peripheral VA ECMO are the most common ECLS technique used in thoracic surgery. Their optimal configurations are well defined and commonly used worldwide.
Venovenous extracorporeal membrane oxygenation (VV ECMO)
The VV ECMO technique needs only peripheral vascular access. This ECLS technique is subdivided in (I) double-site VV ECMO and (II) single-site VV ECMO.
Double-site VV ECMO
Double-site VV ECMO is the most efficient technique for respiratory support, specially in case of high level of O2 requirement. In term of oxygenation, Rich et al., demonstrated the superiority of the drainage of the inferior cava system via a femoral cannula associated with the re-injection in the right atrium via the right internal jugular vein (10).
The double-site VV ECMO insertion is feasible in intensive care unit at the patient bedsite as well as in the operating room. The right internal jugular and the right or the left femoral veins are cannulated percutaneously. The position targets for the outflow and the inflow cannulas are the junction between the right atrium and the inferior vena cava and the superior vena cava, respectively. The intracorporeal length of the outflow cannula may be clinically estimated and corresponds to the length between the skin insertion point of the cannula and the xyphoid process. The correct position of this drainage cannula is ideally guided during the procedure by echocardiography or fluoroscopy. A chest X-ray may also certify the good positioning of the cannulas but is generally done at the end of the procedure once the dressing is realized and the cannulas secured with several stitches. The injection cannula in the right internal jugular vein is usually implanted until his proximal extremity without functional issue.
In term of sizes of the cannulas, it is essential to use large cannulas (e.g., 25 Fr femoral cannula and 24 Fr right internal jugular cannula) with the aim to have an ECMO flow able to reach 8 LPM.
Single-site VV ECMO
VV ECMO can be realized through a single-site using the Avalon Elite™ double-lumen cannula (Avalon Elite™, Maquet Cardiopulmonary GmbH, Rastatt, Germany). This cannula designed by Zwischenberger et al. allows to keep the groins free and is usually implanted in the right internal jugular vein. The outflow lumen allows to drain the blood coming from both the superior vena cava and the inferior vena cava. The injection port is placed directly in the right atrium, in front of the tricuspid valve.
To keep the neck free, Shaffi et al. also described the implantation of this bi-cava cannula in the left subclavian vein. It seems that this site of implantation should be reserved to small patients. It would allow a better mobility of the patients and decrease the rate of local infection in tracheotomised patients (11).
The single-site VV ECMO can be implanted in the intensive care unit as well as in the operating room. The cannulation should be performed under image (transthoracic/transesophageal echocardiography; fluoroscopy) guidance to avoid cardiovascular perforation and to guide the inflow in front of the tricuspid valve (12).
The size of this double-stage cannulas ranges from 13 to 31 Fr. Thus the limiting factor of this dual lumen cannula is the maximal flow available and therefore the oxygenation capacity in case of high level of O2 requirements. Nevertheless the dual lumen cannula was also designed to decrease the process of recirculation.
Venoarterial extracorporeal membrane oxygenation (VA ECMO)
VA ECMO is most commonly performed in a femoro-femoral configuration. This is due to the easy access of the femoral vessels in an emergency context. In case of surgical approach, it is usual to cannulate the femoral vein and the femoral artery from the same side. During percutaneous procedure (e.g., heart catheterization), it can be helpful to use the femoral artery from one side and the femoral vein from the other side. Indeed at the time of ECMO removal, the venous side can be only compressed whereas the dissection of the arterial side will be easier.
The size of the venous cannula should be as large as possible (e.g., 25 Fr). The selection of the size of the injection cannula depends on the calibre of the femoral artery and constitutes a balance between the risk of the inferior limb ischemia and the maximal flow available. It seems that a 21-Fr inflow cannula is an optimal size in this configuration.
Interesting, is the “sport model” describes by Biscotti and Bacchetta corresponding to a right internal jugular vein-right subclavian artery VA ECMO. This model has the advantages of allowing (I) an optimal brain and heart oxygenation and (II) ambulatory activities (13).
ECLS configuration: rationale and results according the indications in thoracic surgery
Acute respiratory distress syndrome (ARDS)
The ARDS is the most severe form of lung injury and the largest indication of ECLS in thoracic surgery. The definition of ARDS has been updated in 2012 by the Berlin definition of the ARDS (14). ECMO is indicated in severe and refractory ARDS in association with the protective ventilatory strategy.
In ARDS, the best ECLS technique to perform is the double-site VV ECMO due to:
- The high level of O2 requirement;
- The absence of heart failure;
- The presence, in the initial phase, of a major systemic inflammatory response syndrome (SIRS) avoiding an ambulatory ECMO strategy.
The clinical results of the VV ECMO in the ARDS are assessing in prospective multicenter trials as the EOLIA trial (15). Currently, the role of the VV ECMO in severe and refractory ARDS is to allow a protective ventilatory strategy and to put the native and injured lungs at rest. The ongoing published data conclude that the patient outcomes are significantly better if the patients cares are performed in ARDS expert centers able to realize ECMO if needed (7,16,17).
Lung transplantation (LTx)
ECLS can be realized at different stages of the LTx process: (I) as bridge to LTx; (II) as an intraoperative support; and (III) after LTx in case of severe primary graft dysfunction (PGD).
ECLS as bridge to lung transplantation (LTx)
ECLS as bridge to LTx is preferentially indicated in patients already listed in a LTx waiting list. The objective of the ECLS is double: (I) restitute the hematosis; and (II) keep the patient in good conditions (awake, wean of mechanical ventilation, ambulatory) for better LTx outcomes.
Excepted for patients suffering from terminal pulmonary hypertension, the best ECLS strategy to bridge patients to LTx is the single-site VV ECMO using the Avalon Elite™ cannula. The rationale of this choice is:
- Usually, a moderate level of O2 requirement;
- The low prevalence of SRIS allowing to avoid or to wean the mechanical ventilation;
- The major clinical interest to keep the patient awake, ambulatory, and active.
The results of ECLS as bridge to LTx has been published by different authors in small series with percentages of transplanted patients from 25% to 100% and 1-year post-LTx survival rates from 75% to 93% (18). In 2013, Lafarge et al. demonstrated a better mid-term survival after ECMO as bridge to LTx in cystic fibrosis patients than those suffering from others indications (19).
The results of the ambulatory strategy have also been recently reported. This strategy allows to significantly decrease (I) the duration of mechanical ventilation after LTx; (II) the ICU length of stay; and (III) the post-LTx hospital length of stay (20,21).
Concerning the pulmonary hypertension patient, VV ECMO should be avoided. Indeed VV ECMO would significantly increase the right ventricule preload and would promote a life-threatening right and left ventricles dysfunction. For these patients the best ECLS strategy is the PA-LA Novalung. The PA-LA Novalung may be preceded and initially associated with a peripheral VA ECMO. The rationale of the PA-LA Novalung as bridge to LTx is to:
- Restitute the hematosis;
- Start the right ventricle remodeling;
- And keep the patients awake and active via an ambulatory strategy (22,23).
The PA-LA Novalung would allow to significantly decrease the mortality rates on LTx waiting list and to enlarge the indication of LTx—instead heart-LTx—in those patients (22,23).
ECLS as an intraoperative support
Central or peripheral VA ECMO can be used during LTx procedures as an intraoperative support. The use of VV ECMO as intraoperative respiratory support is not described.
In case of bilateral or right single LTx, the central aortic and atrial cannulations can be realized through a Clamshell incision or a right hemi-clamshell incision, respectively. In case of left single LTx, to avoid a Clamshell incision, it is possible to cannulate the aorta according a central mode and a femoral vein percutaneously.
Peripheral VA ECMO has the advantage to be kept post-transplantation if needed.
Comparing the CPB, VA ECMO would allow to decrease the requirement for dialysis, the risk of bleeding, the need of blood products transfusions, the rates of PGD, and the ICU and hospital lengths of stay (24-26).
ECLS as bridge to recovery in severe primary lung graft dysfunction
PGD is the leading cause of death early after LTx. PGD represents a multifactorial injury to the transplanted lungs that develops within 72 hours after LTx. PGD is defined and classified by (I) the presence of a bilateral lung edema in absence of heart dysfunction or overfluid status; and (II) the degree of hypoxemia. ECLS is indicated in severe and refractory grade 3 PGD (27).
The ECLS strategy to promote in a PGD context is VV ECMO in absence of circulatory failure. Double-site or single-site VV ECMO have both several advantages and drawbacks. Double-site VV ECMO will allow a better oxygenation in case of high level of O2 requirements whereas the mono-site VV ECMO will be compatible with an ambulatory strategy once the early SIRS stage ends. In case of selection of the single site VV ECMO it might be pertinent to use a 31-Fr cannula.
The results of the ECLS in PGD are still discussed. The best prognosis are described in case of early use of ECMO post-Lx (in the first 48 hours) with survival rates from 50% to 80% (28). Concerning mid-term outcomes after LTx, the ECMO requirement for PGD does not affect the survival and the pulmonary function tests (29).
Conclusions
ECLS represents a large therapeutic arsenal for the thoracic surgeon. The increasing panel of ECLS technologies available is the results of a century of clinical practices, engineering progress, and improvements of accurate physiological knowledges. In front of the technologies, the thoracic surgeon should balance the advantages and the deficiencies of each technique available to treat the patient with the most pertinent and adapted ECLS technology. The selection of the ECLS technique and configuration should answer the following questions:
- What are the current and the future O2 requirements? Which level of flow my ECLS should provide?
- Are the left and the right ventricle functions optimal enough to avoid a circulatory support?
- Is the nosological context associated with a SRIS? Is an ECLS ambulatory strategy practicable?
The nature of the vascular access according the ECLS technique selected is now well defined and responds more to an evidence-based medicine than validated clinical baselines. The selected ECLS technology induces the nature of the vessels used for cannulation. The nature of the cannulated vessels induces the approach strategy.
New technologies and new configurations are going to increase the panel of ECLS technologies available with the aim to (I) improve the overall outcomes; (II) be less invasive and more specific for a given disease; but also (III) enlarge the indication of ECLS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs 1976;22:80-93. [PubMed]
- Marcolin R, Mascheroni D, Pesenti A, et al. Ventilatory impact of partial extracorporeal CO2 removal (PECOR) in ARF patients. ASAIO Trans 1986;32:508-10. [Crossref] [PubMed]
- Kolobow T. Gas exchange with membrane lungs. In: Gille JP, editor. Neonatal and adult respiratory failure: mechanisms and treatment. Paris: Elsevier, 1989:89-96.
- Gattinoni L, Kolobow T, Damia G, et al. Extracorporeal carbon dioxide removal (ECCO2R): a new form of respiratory assistance. Int J Artif Organs 1979;2:183-5. [PubMed]
- Lewandowski K, Slama K, Falke KJ. Approaches to improve survival in severe ARDS. In: Vincent JL, editor. Yearbook of intensive Care and Emergency Medicine. Berlin: Springer Verlag, 1992:372-83.
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Del Sorbo L, Cypel M, Fan E. Extracorporel life support for adults with severe acute respiratory failure. Lancet Respir Med 2014;2:154-64. [Crossref] [PubMed]
- Lund LW, Federspiel WJ. Removing extra CO2 in COPD patients. Curr Respir Care Rep 2013;2:131-8. [Crossref] [PubMed]
- Rich PB, Awad SS, Crotti S, et al. A prospective comparison of atrio-femoral and femoro-atrial flow in adult veno-venous extracorporeal life support. J Thorac Cardiovasc Surg 1998;116:628-32. [Crossref] [PubMed]
- Shaffi AE, McCurry KR. Subclavian insertion of the bicaval dual lumen cannula for venovenous extracorporeal membrane oxugenation. Ann Thorac Surg 2012;94:663-5. [Crossref] [PubMed]
- Javidfar J, Wang D, Zwischenberger JB, et al. Insertion of bicaval dual lumen extracorporeal membrane oxygenation catheter with image guidance. ASAIO J 2011;57:203-5. [Crossref] [PubMed]
- Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487-9. [Crossref] [PubMed]
- ARDS definition task force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526-33.
- Assistance Public-Hôpitaux de Paris. Extracorporeal membrane oxygenation for acute respiratory distress syndrome EOLIA. Available online: http://clinicaltrials.gov/ct2/show/NCT01470703. Accessed April 24, 2014.
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Langer T. Clinical review: extracorporeal membrane oxygenation. Crit Care 2011;15:243. [Crossref] [PubMed]
- Cypel M, Keshavjee S. Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med 2011;32:245-51. [Crossref] [PubMed]
- Lafarge M, Mordant P, Thabut G, et al. Experience of extracorporeal membrane oxygenation as bridge to lung transplantation in France. J Heart Lung Transplant 2013;32:905-13. [Crossref] [PubMed]
- Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55-9. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Strueber M, Hoeper MM, Fischer S, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant 2009;9:853-7. [Crossref] [PubMed]
- De Perrot M, Granton JT, McRae K, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant 2011;30:997-1002. [Crossref] [PubMed]
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Biscotti M, Yang J, Sonett J, et al. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2014;148:2410-6. [Crossref] [PubMed]
- Machuga TN, Collaud S, Mercier O, et al. Outcomes of intraoperative ECMO versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT working group on primary lung graft dysfunction Part II: Definition. A consensus statement of the international society for heart and lung transplantation. J Heart Lung Transplant 2005;24:1454-9. [Crossref] [PubMed]
- Fiser SM, Kron IL, McLendon Long S, et al. Early intervention after severe oxygenation index elevation improves survival following lung transplantation. J Heart Lung Transplant 2001;20:631-6. [Crossref] [PubMed]
- Bermudez CA, Adusumilli PS, McCurry KR, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long term survival. Ann Thorac Surg 2009;87:854-60. [Crossref] [PubMed]


