
The long-term outcomes of surgical ablation for atrial fibrillation during redo left-sided valvular surgery
Highlight box
Key findings
• Concomitant surgical arrhythmia ablation with redo surgery resulted in better overall survival and lower incidence of the composite of thromboembolism and bleeding.
What is known and what is new?
• The effectiveness and safety of the ablation procedure during redo surgery are still controversial;
• The efficacy and safety for surgical ablation in redo surgery are evaluated.
What is the implication, and what should change now?
• Concomitant surgical ablation procedure should be considered in patients undergoing redo cardiac surgery.
Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke and mortality, and the Cox-maze procedure is the standard surgical treatment for AF. Although some studies suggest that the procedure is associated with a higher conversion rate to sinus rhythm and lower risks of mortality and thromboembolism (1-3), the effectiveness and safety of the Cox-maze procedure when performed during redo cardiac surgery are still controversial, given the longer cardiopulmonary bypass and cross-clamp times and higher rates of perioperative morbidities of the Cox- maze procedure (4). Although a few studies that evaluated the effectiveness of the procedure included a redo cardiac surgery group (1,5-8), the redo surgery cases comprised only 10% or less of the cases.
The efficacy and safety for surgical ablation (SA) procedure in patients undergoing redo cardiac surgery are not known. Therefore, this study compared the long-term outcomes of patients who underwent redo cardiac surgery for left-sided valvular heart disease with or without concomitant SA for AF over 10 years. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1018/rc).
Methods
Patient enrollment
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the institutional review board of Seoul National University Hospital as a minimal-risk retrospective study that did not require individual consent (approval No. H-2103-115-1205).
Consecutive patients undergoing redo cardiac surgery for left-sided valvular disease between 2000 and 2015 were included. Patients were excluded if they did not have a history of AF, had undergone SA during a previous procedure, or had a permanent pacemaker.
AF was classified as paroxysmal, persistent, long-standing persistent, and permanent, following established guidelines (9). The SA was indicated in 6 patients with paroxysmal AF, 32 with persistent AF, and 35 with long-standing persistent AF. The decision to perform concomitant arrhythmia ablation was at the surgeon’s discretion considering the patient’s age, number of surgeries, surgical indications, duration of AF and left atrial size.
Operative strategy
Surgical technique and strategy of Cox-maze procedure have been described previously (10). We first performed the Cox-maze III procedure in 2000 using cryoablation, but since 2005, we added bipolar radiofrequency to AF surgery. In this series, cryoablation was used in all cases, and radiofrequency was added in six cases at the surgeon’s discretion. Since 2015, all SAs used cryoablation only. All procedures were performed through a median sternotomy, with aorto-bicaval cannulation under moderate hypothermia and cold cardioplegic arrest. The type of SA was selected under the surgeon’s discretion. Ablation procedures for left atrium only were performed as described in the reference (10).
Evaluation of early and long-term clinical outcomes
Operative mortality was defined as death within 30 days of surgery or during the same hospitalization period. Postoperative low cardiac output syndrome (LCOS) was defined as the need for mechanical or inotropic support to maintain systolic blood pressure >90 mmHg after correcting of reversible factors.
The final rhythm was evaluated from the final electrocardiogram (ECG) at the most recent follow-up. AF, atrial flutter, or pacing rhythm with underlying AF was classified as postoperative AF. Sinus rhythm was defined as a rhythm other than postoperative AF, i.e., normal sinus or junctional rhythm. If the patient developed early postoperative AF or late recurrent AF, the patient was treated with an antiarrhythmic medication.
The patients were followed as outpatients at 3- to 6-month intervals. The patients’ condition was checked by telephone if they did not attend a scheduled visit. In addition, survival data were obtained from the Statistics Korea, a national organization that collects and aggregates data on the people and economy in Korea including 100% complete data on death and cardiac death, through December 31, 2020. The primary endpoint was overall survival.
A composite endpoint of thromboembolism and major bleeding (CTEB) was defined as bleeding or thromboembolic events that caused death, hospitalization or permanent injury, or necessitated a transfusion. Thus, transient ischemic attack was excluded from CTEB events. The clinical follow-up period ended on May 30, 2021.
Statistical analysis
The statistical analyses were performed using SPSS (version 25.0, IBM, Armonk, NY, USA) and R (version 3.6.2). The two groups were compared using Chi-square test or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Survival rates were estimated using the Kaplan-Meier method. Risk factors for all-cause mortality were analyzed using multivariate Cox proportional hazards models. Cumulative incidences of CTEB and reoperation were estimated using all-cause death as a competing risk for the events. The cumulative incidences of each event were compared between the groups using Fine-Gray’s test. Variables included in the multivariate analysis for early and long-term results were age, sex, body surface area, risk factors (smoking, hypertension, diabetes mellitus, body mass index ≥25 kg/m2, history of stroke, chronic kidney disease, coronary artery disease, dyslipidemia, poor functional class, paroxysmal AF, AF duration, patients undergoing more than two operations, preoperative left ventricular dysfunction, and severely dilated left atrium), previous cardiac procedures (aortic, mitral, or tricuspid valve, coronary artery bypass graft, or aorta surgeries) and concomitant procedures (mitral, tricuspid, or aortic valve procedures). Impaired left ventricle was defined as left ventricular ejection fraction less than 50%. The left atrium was diagnosed as severely dilated if its anteroposterior diameter on parasternal long axis view was greater than or equal to 60 mm at end systole. Variables with a P value <0.10 in the univariate analyses were entered into the multivariate models. A P value <0.05 was considered as statistically significant.
Propensity score (PS) analyses were performed to adjust for baseline differences between the groups. The variables in Table 1 in addition to concomitant procedures (mitral, tricuspid, or aortic valve procedures) were entered into logistic regression models to generate a PS. The PS was used as PS-adjusted multivariate models.
Table 1
| Variables | Total (n=224) | SA group (n=73) | NSA group (n=151) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 57.0±11.3 | 54.1±11.3 | 58.4±11.1 | 0.008 |
| Female, n (%) | 142 (63.4) | 42 (57.5) | 100 (66.2) | 0.264 |
| Body surface area (m2), mean ± SD | 1.54±0.17 | 1.58±0.17 | 1.52±0.16 | 0.612 |
| Risk factors, n (%) | ||||
| Smoking | 19 (8.5) | 9 (12.3) | 10 (6.6) | 0.238 |
| Hypertension | 34 (15.2) | 10 (13.7) | 24 (15.9) | 0.818 |
| Diabetes mellitus | 22 (9.8) | 11 (15.1) | 11 (7.3) | 0.111 |
| BMI ≥25 kg/m2 | 25 (11.2) | 11 (15.1) | 14 (9.3) | 0.287 |
| History of stroke | 23 (10.3) | 9 (12.3) | 14 (9.3) | 0.637 |
| CKD | 43 (19.2) | 10 (13.7) | 33 (21.9) | 0.203 |
| Coronary artery disease | 8 (3.6) | 5 (6.8) | 3 (2.0) | 0.146 |
| Dyslipidemia | 7 (3.1) | 4 (5.5) | 3 (2.0) | 0.318 |
| NYHA functional class ≥3 | 117 (52.2) | 34 (46.6) | 83 (55.0) | 0.300 |
| Paroxysmal AF | 13 (5.8) | 6 (8.2) | 7 (4.6) | 0.441 |
| Persistent AF | 76 (33.9) | 32 (43.8) | 44 (29.1) | 0.029 |
| Long-standing persistent AF | 135 (60.3) | 35 (47.9) | 100 (66.2) | 0.013 |
| History of cardiac surgery ≥2 | 56 (25.0) | 6 (8.2) | 50 (32.9) | <0.001 |
| Previous cardiac surgery, n (%) | ||||
| Mitral valve procedure | 218 (97.3) | 70 (95.5) | 148 (97.4) | 0.303 |
| Aortic valve procedure | 77 (34.4) | 11 (15.1) | 66 (43.7) | <0.001 |
| Tricuspid valve procedure | 52 (23.2) | 9 (12.3) | 43 (28.4) | 0.020 |
| CABG | 5 (2.2) | 2 (2.7) | 3 (2.0) | >0.999 |
| Aorta surgery | 8 (3.6) | 3 (4.1) | 5 (3.3) | >0.999 |
| Impaired LV (EF <50%), n (%) | 43 (19.2) | 14 (19.2) | 29 (19.2) | >0.999 |
| Severely dilated LA (size >60 mm), n (%) | 90 (40.2) | 23 (31.5) | 67 (44.4) | 0.090 |
CKD was defined as decreased kidney function (decreased glomerular filtration rate) for 3 or more months, which is adapted from the definition of chronic kidney disease by The Kidney Disease: Improving Global Outcomes Work Group (glomerular filtration rate less than 60 mL/min/1.73 m2). AF, atrial fibrillation; BMI, body mass index; ESRD, end-stage renal disease; LA, left atrium; LV, left ventricle; EF, ejection fraction; NYHA, New York Heart Association; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; SA, surgical ablation; NSA, non-surgical ablation; SD, standard deviation.
Results
Patient characteristics and operative data
From January 2000 to December 2015, 263 consecutive patients underwent redo cardiac surgery for left-sided valvular heart disease under sternotomy, and had preoperative AF at our institution. Those patients who underwent surgery due to active endocarditis (n=24), had a preoperative permanent pacemaker (n=4), or had undergone Cox-maze procedure previously (n=15) were excluded. The median follow-up duration was 124 (1–249.5) months. The median ECG follow-up was 115 (1–246.2) months. Completeness of follow-up was 100% for overall survival and freedom from cardiac death and 95.1% (213 of 224 patients) for other long-term clinical outcomes.
Consequently, the study enrolled 224 patients (57.0±11.3 years; 82 males, 142 females), of whom 73 underwent concomitant SA for AF. SA for AF during redo cardiac surgery for left-sided valvular heart disease was performed in 73 patients (SA group), while the other 151 patients did not undergo surgical arrhythmia ablation (NSA group). Table 1 summarizes the preoperative characteristics. The median duration of AF was 13 (range, 5.5–35.0) years. None of the patients had previous catheter ablation. The NSA group was significantly older, more frequently underwent at least two previous cardiac surgeries, and underwent more previous aortic and tricuspid valve procedures. Patients with long-standing persistent AF were more frequent in the NSA group. Most of the previous surgery procedures were performed before surgical arrhythmia ablation was widespread.
Of 73 patients who underwent surgical arrhythmia ablation, 64 underwent a complete lesion set of Cox-maze III procedure, whereas 9 underwent left-sided SA.
Table 2 summarizes the surgical characteristics of the patients. The mean cardiopulmonary bypass and aortic cross clamp times were 255.4±149.4 and 161.4±51.8 minutes, respectively. The concomitant cardiac procedures performed included mitral (n=210), aortic (n=124), and tricuspid (n=144) valve procedures, coronary artery bypass graft (n=5), and aorta replacement (n=8). The SA group was less likely to undergo a concomitant aortic or tricuspid valve procedure. The aortic cross-clamp time was longer in the SA group but it was not statistically significant.
Table 2
| Variables | Total (n=224) | SA group (n=73) | NSA group (n=151) | P value |
|---|---|---|---|---|
| Concomitant MV procedure, n (%) | 210 (93.8) | 68 (93.2) | 142 (94.0) | 0.368 |
| Replacement | 187 (83.5) | 63 (86.3) | 124 (82.1) | |
| Repair | 4 (1.8) | 2 (2.7) | 2 (1.3) | |
| Others (pannus removal etc.) | 19 (8.5) | 3 (4.1) | 16 (10.5) | |
| Concomitant AV procedure, n (%) | 124 (55.4) | 36 (49.3) | 88 (58.3) | 0.040 |
| Replacement | 110 (49.1) | 28 (38.4) | 82 (54.3) | |
| Repair | 9 (4.0) | 6 (8.2) | 3 (2.0) | |
| Others (pannus removal etc.) | 5 (2.2) | 2 (2.7) | 3 (2.0) | |
| Concomitant TV procedure, n (%) | 144 (64.3) | 47 (64.4) | 97 (64.2) | >0.999 |
| Replacement | 40 (17.9) | 7 (9.6) | 33 (21.9) | |
| Repair | 99 (44.2) | 40 (54.8) | 59 (39.1) | |
| Others (pannus removal etc.) | 5 (2.2) | 0 (0.0) | 5 (3.3) | |
| Tissue valve implantation, n (%) | 38 (17.0) | 11 (15.1) | 27 (17.9) | |
| Concomitant CABG, n (%) | 5 (2.2) | 2 (2.7) | 3 (2.0) | 0.662 |
| Concomitant aorta surgery, n (%) | 8 (3.6) | 3 (4.1) | 5 (3.3) | 0.718 |
| CPB time (minutes), mean ± SD | 255.4±149.4 | 243.4±58.3 | 261.2±177.3 | 0.266 |
| ACC time (minutes), mean ± SD | 161.4±51.8 | 169.7±43.4 | 157.4±55.1 | 0.071 |
MV, mitral valve; AV, aortic valve; TV, tricuspid valve; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross clamp; SD, standard deviation; SA, surgical ablation; NSA, non-surgical ablation.
Early results
Operative mortality occurred in 18 patients [8.0%; 4 (5.5%) in the SA group and 14 (9.3%) in the NSA group]; the difference between groups was not significant (P=0.474). Postoperative complications included LCOS (n=44, 19.6%), respiratory complications (n=28, 12.5%), acute renal failure (n=27, 12.1%), postoperative bleeding requiring reoperation (n=16, 7.1%), stroke (n=14, 6.3%), and mediastinitis (n=5, 2.2%). The NSA group had a higher rate of postoperative LCOS compared with the SA group [36 (23.8%) vs. 8 (11.0%), P=0.036; Table 3]. There were no significant differences in the incidences of other postoperative complications between the two groups. In multivariate analyses, SA for AF was not associated with operative mortality (Table S1). Concomitant SA for AF was associated with a lower rate of postoperative LCOS in the multivariate analysis [odds ratio, 0.328; 95% confidence interval (CI): 0.136–0.788, Table S2]. None of the patients had newly implanted pacemakers during the hospital stay after the SA procedure. During the follow-up, a total of 5 patients had newly implanted pacemakers (4 from NSA group and 1 from SA group). There was no significant difference in the incidence of postoperative complications between those with sinus rhythm restoration and not.
Table 3
| Variables | Total (n=224) | SA group (n=73) | NSA group (n=151) | P value |
|---|---|---|---|---|
| Operative mortality, n (%) | 18 (8.0) | 4 (5.5) | 14 (9.3) | 0.474 |
| Complications, n (%) | 86 (38.4) | 21 (28.8) | 65 (43.0) | 0.056 |
| LCOS | 44 (19.6) | 8 (11.0) | 36 (23.8) | 0.036 |
| Respiratory complication | 28 (12.5) | 8 (11.0) | 20 (13.2) | 0.788 |
| Acute kidney injury | 27 (12.1) | 5 (6.8) | 22 (14.6) | 0.149 |
| Bleeding reoperation | 16 (7.1) | 4 (5.5) | 12 (7.9) | 0.693 |
| Stroke | 14 (6.3) | 2 (2.7) | 12 (7.9) | 0.225 |
| Mediastinitis | 5 (2.2) | 1 (1.4) | 4 (2.6) | 0.901 |
LCOS, low cardiac output syndrome; SA, surgical ablation; NSA, non-surgical ablation.
Long-term survival
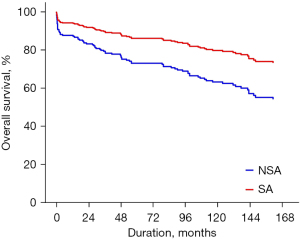
Late death occurred in 81 patients, including cardiac deaths in 41. The 10- and 15-year overall survival rates were 67.6% and 58.7%, respectively. The 10- and 15-year rates of freedom from cardiac death were 83.4% and 76.9%, respectively. Overall survival was better in the SA group (P<0.001, Figure 1). Even after risk factor adjustment, the PS-adjusted multivariate analysis showed that concomitant surgical arrhythmia ablation was significantly associated with overall survival [reference, NSA group; hazard ratio (HR), 0.452; 95% CI: 0.218–0.936; Table 4 and Figure 2]. Older age at operation, poor functional class (New York Heart Association functional class III or IV), and concomitant tricuspid valve procedure were significant risk factors for overall mortality (Table 4).
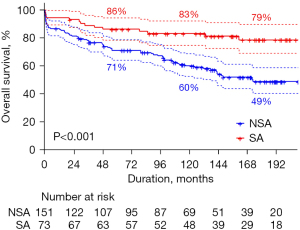
Table 4
| Variables | Factors associated with overall survival | ||||
|---|---|---|---|---|---|
| Univariate analysis | PS-adjusted multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Surgical arrhythmia ablation | 0.369 (0.207–0.656) | <0.001 | 0.452 (0.218–0.936) | 0.032 | |
| Age (years) | 1.079 (1.055–1.104) | <0.001 | 1.071 (1.044–1.098) | <0.001 | |
| NYHA functional class ≥ III | 2.207 (1.384–3.520) | <0.001 | 1.719(1.060–2.787) | 0.028 | |
| Concomitant TV operation | 1.759 (1.070–2.891) | 0.026 | 1.910 (1.150–3.171) | 0.012 | |
| CKD | 1.944 (1.195–3.160) | 0.007 | 1.279 (0.763–2.142) | 0.351 | |
| Long-standing persistent AF | 1.812 (1.129–2.907) | 0.014 | 1.107 (0.664–1.845) | 0.697 | |
| Giant LA | 1.676 (1.082–2.596) | 0.021 | 1.038 (0.650–1.658) | 0.876 | |
HR, hazard ratio; CI, confidence interval; PS, propensity score; TV, tricuspid valve; CKD, chronic kidney disease; AF, atrial fibrillation; LA, left atrium, NYHA, New York heart association
Recurrence of AF
In SA group, 49 patients had postoperative sinus rhythm whereas 6 patients had postoperative sinus rhythm in NSA group. The maintenance of postoperative sinus rhythm at the final ECG was detected in 35 (47.9%) patients in the SA group, while 16 (10.5%) in the NSA group had postoperative sinus rhythm at the final ECG with medical treatment. None of the patients in the SA group underwent radiofrequency catheter ablation. Of the 16 patients, 10 (6.6%) had sinus rhythm and 6 (4.0%) had junctional rhythm. In the SA group, 30 (41.1%) patients had sinus rhythm and 5 (6.8%) junctional rhythm. Most of the patients (93.3%) used antiarrhythmic drug (e.g., amiodarone, most commonly) except those with side effects on antiarrhythmic drug.
In the competing risk analysis of AF recurrence, the cumulative incidence differed significantly between the two groups (P<0.001, Figure 3). Even after adjusting for the PS, concomitant surgical arrhythmia ablation was a significant factor in the PS-adjusted multivariate analysis (reference, NSA group; HR, 0.505; 95% CI: 0.369–0.691). Besides concomitant SA, the PS-adjusted multivariate analysis showed that multiple redo operation (P=0.043) was marginally associated with recurrent AF (Table 5).
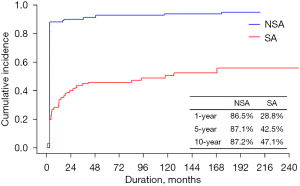
Table 5
| Variables | Factors associated with atrial fibrillation | ||||
|---|---|---|---|---|---|
| Univariate analysis | PS-adjusted multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Surgical arrhythmia ablation | 0.448 (0.335–0.600) | <0.001 | 0.505 (0.369–0.691) | <0.001 | |
| Paroxysmal AF | 0.449 (0.206–0.982) | 0.045 | 0.490 (0.224–1.070) | 0.074 | |
| >2nd redo operation | 1.440 (1.180–1.760) | <0.001 | 1.216 (1.006–1.470) | 0.043 | |
| Previous AV procedure | 1.170 (1.020–1.330) | 0.021 | 1.050 (0.920–1.198) | 0.470 | |
| Age (years) | 1.010 (1.000–1.020) | 0.014 | 1.005 (0.996–1.014) | 0.311 | |
| Long-standing persistent AF | 0.764 (0.606–0.963) | 0.023 | 0.864 (0.686–1.089) | 0.215 | |
HR, hazard ratio; CI, confidence interval; PS, propensity score; AF, atrial fibrillation; AV, aortic valve.
For the patients with sinus rhythm for a certain period of time with bioprosthetic valves, anticoagulation was ceased. The cessation of anticoagulation was decided based on multiple intermittent ECG recordings. Twenty-seven patients (17.9%) underwent bioprosthetic valve replacement in NSA group and 11 patients (15.1%) underwent tissue valve replacement in the SA group (P=0.705). Out of these 11 patients, 3 patients restored sinus rhythm and all of them ceased anticoagulation. As most of the patients (84.9%) in the SA group underwent mechanical valve replacement, anticoagulation could not be ceased even in non-AF patients.
Composite of thromboembolism and bleeding events
CTEB events occurred in 7 (9.6%) patients in the SA group and 27 (17.9%) in the NSA group. Major bleeding events occurred in 6 (8.2%) and 16 (10.6%) patients in the SA and NSA groups, respectively. In all of the patients with bleeding or thromboembolic event, international normalized ratio (INR) was in therapeutic range. The incidence of thromboembolism events did not show significant difference [12 (7.9%) vs. 1 (1.4%); P=0.095].
The cumulative incidence of CTEB at 10 years was 8.3% in the SA group and 13.1% in the NSA group; the difference was significant (P=0.037, Figure 4). The PS-adjusted multivariate analysis also showed a lower incidence of CTEB in the SA group (reference, NSA; HR, 0.338; 95% CI: 0.127–0.897). In the PS-adjusted multivariate analysis, previous tricuspid valve surgery (P=0.017) and older age (P=0.006) were also associated with CTEB (Figure 4 and Table 6).
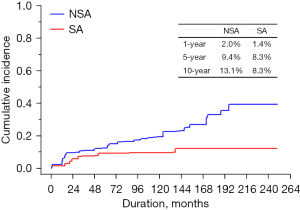
Table 6
| Variables | Factors associated with CTEB | ||||
|---|---|---|---|---|---|
| Univariate analysis | PS-adjusted multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Surgical arrhythmia ablation | 0.411 (0.179–0.947) | 0.037 | 0.338 (0.127–0.897) | 0.029 | |
| Previous TV operation | 2.080 (1.040–4.170) | 0.039 | 2.710 (1.191–6.165) | 0.017 | |
| >2nd redo operation | 0.307 (0.093–1.012) | 0.052 | 0.263 (0.072–0.957) | 0.043 | |
| Age (years) | 1.058 (1.022–1.096) | <0.001 | 1.056 (1.016–1.098) | 0.006 | |
| Long-standing persistent AF | 2.590 (1.159–5.787) | 0.020 | 2.428 (0.985–5.986) | 0.054 | |
| NYHA functional class ≥ III | 2.142 (1.032–4.445) | 0.041 | 1.837 (0.869–3.883) | 0.112 | |
CTEB, composite of thromboembolism and bleeding events; HR, hazard ratio; CI, confidence interval; PS, propensity score; AF, atrial fibrillation; TV, tricuspid valve; NYHA, New York Heart Association.
Discussion
The present study demonstrated three major findings. First, overall survival was better in patients who underwent redo cardiac surgery for left-sided heart disease with versus without concomitant surgical arrhythmia ablation. Second, those with the concomitant surgical arrhythmia ablation had a significantly higher sinus conversion rate compared with those without. Finally, concomitant surgical arrhythmia ablation with redo cardiac surgery was associated with significantly lower cumulative incidence of CTEB.
The Cox-maze procedure is the standard surgical treatment for AF (11,12). However, as the concomitant Cox-maze procedure is more time consuming and complex, it is not clear whether to perform the procedure during redo cardiac surgery for persistent or long-standing persistent AF (13-15). There are only a few reports on the efficacy of the Cox-maze procedure concomitant with redo cardiac surgery (7,8). Stulak et al. (8) focused on the outcomes after redo cardiac operation in patients with congenital heart disease, in which the genesis of AF might differ from that in our cohort. Kobayachi et al. (7) reported the outcomes after redo mitral valve operations in 42 patients: 29 patients in the Cox-maze procedure group and 13 controls. Other studies showed the outcomes of the concomitant procedure during redo cardiac surgery, but in most of those studies, only a small proportion of the subjects underwent previous cardiac surgery (1,5).
As reoperative cardiac surgery is usually associated with higher mortality and morbidity immediately after operation, one of the main concerns is related to early clinical outcomes. In this study, although aortic cross-clamp time was approximately 15 minutes longer in the SA group than in NSA group, the difference was not statistically significant and the early clinical outcomes were comparable, including operative mortality, acute renal failure and reoperation for bleeding; the incidence of LCOS was rather higher in NSA group. Although the higher proportion of high-risk redo surgery in the NSA group could affect these results, concomitant SA for AF was not associated with operative mortality in the multivariate analysis. Moreover, in the multivariate analysis, concomitant SA for AF protected against postoperative LCOS.
In addition to perioperative mortality and morbidity, our data showed that concomitant SA for AF was associated with a low mortality rate, while offering a lower rate of recurrent AF and lower risk of CTEB during follow-up. Although there may be concerns regarding the procedure’s invasiveness and potential morbidity, our data demonstrate that more aggressive concomitant procedures should be considered in select patients.
In our cohort, freedom from AF was relatively low compared with other studies, with recurrent AF rates at last follow-up of 85–97% (16). However, those studies included patients undergoing primary cardiac surgery. The freedom of AF in our study is similar to that of Kobayashi et al. (7), who found that sinus conversion after the Cox-maze procedure was less frequent after redo mitral valve operation than after the primary mitral valve operation. Although a low sinus conversion rate can be problematic because sinus rhythm is related to a reduced incidence of thromboembolic events, our data showed that the Cox-maze procedure decreased the cumulative incidence of thromboembolic events. Moreover, AF is reported to increase mortality risk over the years following the operation (17,18). Thus, the maintenance of sinus rhythm can improve overall survival (4), which is consistent with our result showing better survival after the Cox-maze procedure.
Limitations
There were several limitations to the present study that must be noted. First, this study is limited by its retrospective design. Second, PS matching was unavailable due to relatively small number of patients and heterogeneity of the patients. Third, we performed ECG, which is only a snapshot, rather than Holter monitoring to determine whether sinus rhythm was restored. Fourth, the indication to perform SA during redo surgery was under the surgeon’s discretion. Fifth, preoperative mortality risk scores could not be included in the analysis because patients who underwent surgery in the early study period did not have sufficient data to calculate the scores.
Conclusions
In conclusion, the present study showed that concomitant surgical arrhythmia ablation with redo cardiac surgery for left-sided heart disease resulted in a better overall survival, lower incidence of recurrent AF, and lower incidence of CTEB. Concomitant SA procedure may be considered in patients undergoing redo cardiac surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1018/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1018/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1018/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the institutional review board of Seoul National University Hospital as a minimal-risk retrospective study that did not require individual consent (approval No. H-2103-115-1205).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Louagie Y, Buche M, Eucher P, et al. Improved patient survival with concomitant Cox Maze III procedure compared with heart surgery alone. Ann Thorac Surg 2009;87:440-6. [Crossref] [PubMed]
- Handa N, Schaff HV, Morris JJ, et al. Outcome of valve repair and the Cox maze procedure for mitral regurgitation and associated atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:628-35. [Crossref] [PubMed]
- Bando K, Kobayashi J, Kosakai Y, et al. Impact of Cox maze procedure on outcome in patients with atrial fibrillation and mitral valve disease. J Thorac Cardiovasc Surg 2002;124:575-83. [Crossref] [PubMed]
- Musharbash FN, Schill MR, Sinn LA, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg 2018;155:159-70. [Crossref] [PubMed]
- Gillinov AM, Sirak J, Blackstone EH, et al. The Cox maze procedure in mitral valve disease: predictors of recurrent atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:1653-60. [Crossref] [PubMed]
- Schaff HV, Dearani JA, Daly RC, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg 2000;12:30-7. [Crossref] [PubMed]
- Kobayashi J, Kosakai Y, Isobe F, et al. Rationale of the Cox maze procedure for atrial fibrillation during redo mitral valve operations. J Thorac Cardiovasc Surg 1996;112:1216-21; discussion 1222. [Crossref] [PubMed]
- Stulak JM, Dearani JA, Burkhart HM, et al. The surgical treatment of concomitant atrial arrhythmias during redo cardiac operations. Ann Thorac Surg 2012;94:1894-9; discussion 1900. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-51. [Crossref] [PubMed]
- Kim KC, Cho KR, Kim YJ, et al. Long-term results of the Cox-Maze III procedure for persistent atrial fibrillation associated with rheumatic mitral valve disease: 10-year experience. Eur J Cardiothorac Surg 2007;31:261-6. [Crossref] [PubMed]
- Pinho-Gomes AC, Amorim MJ, Oliveira SM, et al. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg 2014;46:167-78. [Crossref] [PubMed]
- Ad N. The Cox-Maze procedure: history, results, and predictors for failure. J Interv Card Electrophysiol 2007;20:65-71. [Crossref] [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [Crossref] [PubMed]
- Lapenna E, De Bonis M, Giambuzzi I, et al. Long-term Outcomes of Stand-Alone Maze IV for Persistent or Long-standing Persistent Atrial Fibrillation. Ann Thorac Surg 2020;109:124-31. [Crossref] [PubMed]
- Burkhardt JD, Di Biase L, Natale A. Long-standing persistent atrial fibrillation: the metastatic cancer of electrophysiology. J Am Coll Cardiol 2012;60:1930-2. [Crossref] [PubMed]
- Stulak JM, Sundt TM 3rd, Dearani JA, et al. Ten-year experience with the Cox-maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg 2007;83:1319-24. [Crossref] [PubMed]
- Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol 2002;40:84-92. [Crossref] [PubMed]
- Wang CT, Zhang L, Qin T, et al. Cox-maze III procedure for atrial fibrillation during valve surgery: a single institution experience. J Cardiothorac Surg 2020;15:111. [Crossref] [PubMed]


