
miR-183-5p regulates ECM and EMT to promote non-small cell lung cancer progression by targeting LOXL4
Highlight box
Key findings
• miR-183-5p regulated A549 cell proliferation, apoptosis, migration, invasion, ECM accumulation, and EMT by suppressing LOXL4 expression.
What is known and what is new?
• Studies have reported that miR-183-5p plays an importment role in cancers.
• miR-183-5p weakens the tumorigenicity by targeting LOXL4 in non-small cell lung cancer (NSCLC).
What is the implication, and what should change now?
• miR-183-5p/ LOXL4 may be considered as targets for new drugs to treating NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) is a common human cancer, and is estimated to account for >84% of total lung cancer cases worldwide (1-3). The histology of NSCLC can be categorized into two main subtypes: lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (2). Previous studies have shown the development of NSCLC is collectively associated with multiple risk factors, such as tobacco smoking, and inherited genetic mutations, as well as exposure to environmental carcinogens such as radon, asbestos, arsenic compounds, and mustard gas (2,4). Surgery, adjuvant therapy, chemotherapy, and radiotherapy have been the most widely applied methods for treating NSCLC in recent decades (2,4). The initiation and pathogenic progression of NSCLC are driven by a series of gene mutations, epigenetic modifications, immune alterations, and metabolic events that involve the Kirsten rat sarcoma (KRAS) gene, epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and histone deacetylases (HDACs) (1,2,5). As a result of this knowledge, targeted treatments and immunotherapy regimens are now used to treat NSCLC patients (3,5-7). However, the widespread acquisition of multiple resistance to targeted treatments has become a new obstacle for achieving an ideal outcome of clinical treatment (8,9).
In recent years, epigenetic aberrations and chromatin status have been identified as essential players in cancer initiation and progression (10,11). Epigenetic alterations substantially contribute to the pathogenesis of NSCLC by regulating pleiotropic cellular processes and signaling pathways such as the epithelial mesenchymal transition (EMT) pathway (12-14). MicroRNAs (miRNAs) are non-coding RNA molecules consisting of approximately 21 nucleotides, and are critical epigenetic components due to their potent ability to induce the degradation or inhibit the translation of specific target mRNAs (15). Due to their widespread involvement in regulating gene expression, miRNAs play essential roles in various cellular events associated with cancer development and progression, such as the EMT process (16-18), and their pathogenic roles during NSCLC development have also been revealed by extensive research conducted in recent decades (19,20). For example, miR-21, as one of the most intensively studied oncogenic miRNAs associated with NSCLC pathogenesis, can possibly be used for purposes of NSCLC diagnosis and treatment (19). The proliferation, survival, migration, and chemotherapy resistance of cancers were also recently shown to be regulated by many other miRNAs, such as miR-421, miR-409-3p, and miR-195, suggesting key roles for miRNAs in NSCLC pathogenesis (21-23). In addition, miR-183-5p was reported to be differentially expressed in NSCLC tissues and to modulate cancer growth and metastasis (24,25). However, little is known about the target genes of miR-183-5p and cellular mechanisms that regulate NSCLC progression.
Lysyl oxidase-like 4 (LOXL4) belongs to the LOX (lysyl oxidase) protein family, which mainly consists of copper-dependent monoamine oxidase enzymes found in extracellular spaces and catalyze the oxidative deamination of collagen and elastin lysine residues. These enzymes are critical for the production of soluble collagen and elastin crosslinking occurring during extracellular matrix (ECM) remodeling (26,27). The remodeling of ECM plays an essential role in allowing epithelial cell detachment from the basement membrane, which further promotes EMT progression and cancer metastasis (28,29). Hence alterations of ECM components such as collagen type I alpha1 chain (COL1A1) and fibronectin 1 (FN1) are commonly observed in cancer cells of different origins (30). LOX and its four isozymes (LOXL1-4) have also been reported to exert key effects on the proliferation, migration, and metastasis of cancer cells by regulating ECM and EMT processes (26,27). For instance, the stabilization and deposition of collagens in ECM induced by LOXL2 contribute to the migration and metastasis of lung cancer (31).
Changes in LOXL4 expression can also alter the proliferation, migration, invasion, and cycle progression of NSCLC cells (30,32). More importantly, the expression of LOXL4 during NSCLC development was found to be targeted by multiple miRNAs such as miR-135a-5p, miR-210 (30,32) and miR-328-5p (33). However, the possibility of a direct interaction between miR-183-5p and LOXL4 and its effect on cancer development has not been studied. In the present study, we used cellular and animal models to test our hypothesis that miR-183-5p might regulate NSCLC development and progression by targeting expression of the LOXL4 gene. The potential effects of miR-183-5p/LOXL4 interaction on ECM remodeling and EMT progression were further assessed in the context of NSCLC. Our findings provide new insights into the pathogenic mechanisms that drive NSCLC development and progression. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-329/rc).
Methods
Clinical sample collection
Samples of lung cancer tissue and adjacent normal lung tissue were surgically collected from 30 patients diagnosed and treated with surgical segments in the Department of Respiratory Medicine, Xiangya Hospital, Central South University (Changsha, China) between March 5, 2019 and August 8, 2020. The study protocol was approved in advance by the Medical Ethical Committee of Xiangya Hospital (No. 202110993), and written informed consent for study participation was obtained from each patient prior to surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The collected samples of NSCLC and adjacent non-cancerous lung tissue were immediately placed in liquid nitrogen for subsequent analysis.
Cell culture and transfection
Normal human bronchial epithelial cells and human NSCLC cell lines A549, 95D, H1299, and H1650 were obtained from the cell bank affiliated with the Chinese Academy of Sciences (Shanghai, China). The short tandem repeat (STR) method was used for cell line verification. All cell lines were cultured in DMEM (Dulbecco’s Modified Eagle Medium; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (fetal bovine serum) at 37 ℃ in a standard humidified cell culture chamber with 5% CO2. miR-183-5P mimics, an miR 183 5P inhibitor, and si-LOXL4 (5'-AGACUUUCCUGUAGUAGUGGC-3') were synthesized by the RiboBio Company (Guangzhou, China) and transfected into cells using Lipofectamine 3000 Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The LOXL4 gene coding sequences were amplified by PCR, ligated with pcDNA 3.1 plasmids, then transfected into lung cancer cells as described above.
Reverse transcription and microRNA quantitation
For quantitation of miR-183-5p expression, total RNA was extracted from clinical samples of lung tissue, cultured cell lines, and mouse tissues using a Trizol kit (#9109; TAKARA, Tokyo, Japan) according to the manufacturer’s instructions. After determining the RNA concentration in the extract, a 2 ug sample of total RNA from each extract was reverse transcribed into cDNA using a Bestar qPCR RT kit (#2220; DBI, Shanghai, China) as instructed by the manufacturer. Relative levels of miR-183-5p expression were subsequently detected via real-time quantitative PCR performed using a Bestar qPCR MasterMix kit (#2043; DBI) according to the manufacturer’s instructions. U6 expression served as an internal standard for microRNA quantitation, which was performed using the 2−∆∆Ct method. The sequences of primers used for miR-183-5p detection are shown in Table S1.
Western blotting
The total proteins were extracted from cultured NSCLC cell lines, clinical lung cancer tissues, and mouse tumor tissues using a ProteoPrep Total Extraction Sample Kit (#PROTTOT-1KT; Merck Millipore, Billerica, MA, USA) as instructed by the manufacturer. The total protein concentration in extract was measured using the BCA method. Proteins were then denatured by boiling at 100 ℃ for 5 min, after which an equal amount of total protein from each sample (2 µg/µL, 15 µL) was separated by 12% SDS-PAGE. The separated protein bands were then transferred onto PVDF membranes (Merck Millipore), which were subsequently blocked with 5% BSA solution for 2 h at room temperature. The PVDF membranes were then incubated with primary antibodies dilated as appropriate for 2–3 h at room temperature, after which the membranes were incubated with secondary antibodies for 1–2 h at room temperature. A Pierce ECL Western Blotting Substrate kit (#32106; Thermo Fisher Scientific) was used to detect the immunostained protein bands, and GAPDH protein expression served as an internal standard. The primary antibodies used for Western blotting included anti-LOXL4 (#ab88186), anti-beta Catenin (#ab32572), anti-Vimentin (#ab92547), and anti-GAPDH (#ab8245) (all purchased from Abcam, Cambridge, MA, USA), and anti-N-cadherin (#2131), E-cadherin (#3236), anti-LOX (#58135), anti-COL1A1 (#72026), and anti-Fn (#26836), (all purchased from CST, Danvers, MA, USA).
Immunofluorescence (IF) and immunohistochemistry (IHC)
To assess LOXL4 protein expression, cultured NSCLC cells grown on glass slides or sections of mouse tumor tissue (5 um-thick) were fixed with 4% formaldehyde for 25 min at room temperature. Cells and tumor sections were then blocked with 5% BSA solution for approximately 1 h, incubated overnight with anti-LOXL4 antibodies (#ab88186; Abcam; 1:500) or anti-Ki-67 (#ab15580; Abcam; 1:500) at 4 ℃, then incubated in the dark with Alexa Fluor-conjugated anti-Rabbit IgG H&L antibodies (#ab150077; Abcam) for 45 min at room temperature. After counter-staining with DAPI solution, LOXL4 expression in NSCLC cells was assessed by observation under a fluorescence microscope.
Dual luciferase reporter assay
A commercially available dual luciferase reporter assay kit (#E1910; Merck Millipore) was used to verify the binding of miR-183-5p to the promoter region of the LOXL4 gene in NSCLC cells according to instructions provided by the manufacturer. Briefly, the wild-type (WT) and mutant (MUT) versions of LOXL4 promoter sequences were ligated with the pGl4 luciferase reporter vectors then transfected into NSCLC cells using Lipofectamine 3000 Reagent as described above. NSCLC cells were simultaneously transfected with miR-183-5p mimics and/or inhibitors. After cell lysis, the luciferase activity of transfected NSCLC cells was detected with a GloMax luminometer (Promega, Madison, WI, USA).
Cell proliferation
Cell Counting Kit-8 (CCK-8) and Edu staining methods were used to detect the proliferation rates of NSCLC cells. CCK-8 assays were performed using a commercial kit (#CK04; Dojindo, Japan) as instructed by the manufacturer. Briefly, NSCLC cells were cultured in 96-well plates (Corning, Inc., Corning, NY, USA) at 37 ℃ for 24, 48, and 72 h, respectively, then mixed with 10 uL of CCK-8 solution and cultured at 37 ℃ for an additional 2.5 h. Finally, the OD450 (absorbance at 450 nm) value of each culture well was measured using a plate reader. The Edu staining of NSCLC cells was performed using a commercial kit (#ab219801; Abcam) according to the manufacturer’s instructions. Briefly, cultured NSCLC cells were incubated at room temperature with EdU solution (10 µM) for 12 h, after which they were fixed for 15 min in the dark at room temperature, permeabilized in the dark for 12 min, then incubated in the dark with the Reaction mixture and Hoechst 33342 (Beyotime, Shanghai, China) solution for 20 min. Cells were then observed with a confocal microscope.
Cell cycle and apoptosis
The cell cycle progression of NSCLC cells was examined using a Tall Cell Cycle Analysis Kit (#A10798; Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, cultured NSCLC cells were mixed with 10 uL of Cell Cycle solution then incubated in the dark for 20 min at 37 ℃, before being analyzed by flow cytometry. A Dead Cell Apoptosis Kit (#V13242; Thermo Fisher Scientific) was used to analyze NSCLC cell apoptosis according to the manufacturer’s instructions. Briefly, 1.5×105 NSCLC cells resuspended in annexin solution were mixed with 1× annexin V FITC solution and 1 µg/mL of PI solution then incubated in the dark for 15 min at room temperature. The percentages of apoptotic NSCLC cells were then determined by flow cytometry.
Cell migration and invasion
The migration and invasion capabilities of NSCLC cells were analyzed using Transwell assay (Corning) as described in the manufacturer’s instructions. Briefly, NSCLC cells were seeded into the upper chambers of Transwell plates filled with serum-free DMEM then incubated for approximately 48 h. After incubation, cells that had migrated into the lower chambers were stained with 0.01% crystal violet solution for 15 min then observed and counted under a microscope. For analysis of cell invasion, the same procedures were used as described above, except that the inner sides of the upper Transwell chambers were pre-coated with Matrigel (Corning). NSCLC cell migration was also analyzed by wound healing assay. Briefly, NSCLC cells were cultured in 6-well plates for approximately 48 h, after which a scratch line was created in the middle of the NSCLC cell mono-layer with a sterilized needle. The narrowing of the wound line in each culture well was then closely observed and photographed under a microscope.
Cell line-based xenograft model
The tumorigenesis of NSCLC cells was evaluated in vivo using a cell line-based xenograft nude mouse model. A protocol was prepared before the study without registration. All experimental procedures performed with mice were approved by the Experimental Animal Ethics Committee of Xiangya Medical College, Central South University (No. No. 202110993), in compliance with institutional guidelines for the care and use of animals. Briefly, 10 female BALB/c mice (age =6 weeks, 20–25 g) were randomly assigned to a negative control group (NC, n=5) or an miR-183-5p mimics group (n=5), and both were raised in specific pathogen-free animal rooms at 25 ℃ with 50% humidity. NSCLC cells transfected with the miR-183-5p mimics (1×106) were collected, resuspended in PBS, then subcutaneously injected into the nude mice. The formation of tumor xenografts in the mice was closely observed for one month after cell injection. LOXL4 and Ki-67 expression in the tumor tissues was detected by IHC as described above, and the apoptosis of tumor cells was analyzed by TUNEL staining (#ab66110; Abcam) performed according to the manufacturer’s instructions.
Statistical analysis
All data were analyzed using IBM SPSS Statistics for Windows, Version 20 software, and results represent the mean value ± standard deviation of data obtained from at least three independent experiments. Differences between two groups were analyzed by the Student t-test, and differences between more than two groups were analyzed by ANOVA (analysis of variance). A P value <0.05 was considered statistically significant.
Results
miR-183-5p expression negatively correlated with LOXL4 expression in NSCLC tissues
To investigate the pathogenic roles of miR-183-5p and LOXL4 in NSCLC development, we first analyzed the changes in miR-183-5p and LOXL4 expression occurring in the cancerous tissues and adjacent non-cancerous lung tissues collected from 30 NSCLC patients. Quantitative RT-PCR data showed the levels of miR-183-5p expression in the cancerous tissues were significantly lower than in the corresponding non-cancerous lung tissues (Figure 1A). In contrast, the levels of LOXL4 mRNA in cancerous tissues were much higher than in adjacent non-cancerous lung tissues (Figure 1B). Moreover, Western blot results confirmed the levels of LOXL4 protein in the cancerous tissues were significantly elevated when compared with adjacent non-cancerous tissues (Figure 1C). These results indicated the levels of miR-183-5p expression in NSCLC tissues were negatively correlated with LOXL4 expression.
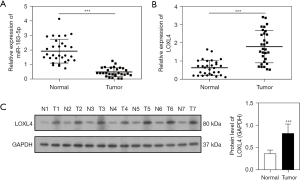
miR-183-5p targeted and suppressed LOXL4 expression in NSCLC cells
The binding site between miR-183-5p and LOXL4 3’UTR is shown in the TargetScan Database (Figure 2A). To study the specific interaction between miR-183-5p and LOXL4 in NSCLC cells, we analyzed the expression of miR-183-5p in multiple NSCLC cell lines. Our results revealed the levels of miR-183-5p expression in the 95D, A549, H1299, and H1650 cells were all significantly lower than in human bronchial epithelial cells (HBEs) (Figure 2B). However, the levels of LOXL4 protein in the 95D, A549, H1299, and H1650 cells were all significantly higher than in the HBE cell line, which further confirmed the negative correlation between miR-183-5p and LOXL4 expression in the context of NSCLC (Figure 2C). Moreover, a dual luciferase reporter assay showed treatment with miR-183-5p mimics greatly suppressed luciferase activity in A549 cells expressing wild type LOXL4 promoter sequences but did not suppress luciferase activity in A549 cells with mutant LOXL4 promoter sequences (Figure 2D). Subsequently, we altered the expressional levels of miR-183-5p in A549 cells by transfection with specific mimics or the inhibitor and found transfection caused a great increase or decrease in miR-183-5p expression (Figure 2E). Interestingly, we also found the levels of LOXL4 gene expression in A549 cells were significantly increased by the miR-183-5p inhibitor, and remarkably repressed by the miR-183-5p mimics, when compared with the negative control group (Figure 2F). These results indicated miR-183-5p could suppress LOXL4 expression in NSCLC cells by directly binding to the LOXL4 gene promoter region.
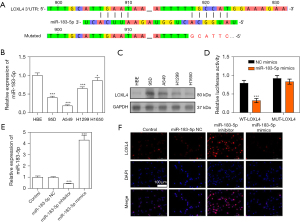
LOXL4 promoted the proliferation and suppressed the apoptosis of A549 cells
To assess the pathogenic roles of LOXL4 in NSCLC development, we altered expression of the LOXL4 gene in A549 cells by transfecting them with an overexpression vector or specific siRNA (Figure 3A). Our data showed LOXL4 expression was greatly elevated by the overexpressing vector and repressed by LOXL4 siRNA, when compared with the negative controls (Figure 3B). Moreover, CCK-8 assays showed the proliferation of A549 cells was significantly promoted by overexpression of the LOXL4 gene but greatly repressed by treatment with LOXL4 siRNA (Figure 3C). The changes in A549 cell proliferation induced by LOXL4 overexpression or knockdown were further validated by the results of Edu staining (Figure 3D). Moreover, we found the percentage of G1-stage A549 cells was decreased and the percentage of G2-stage A549 cells was increased by LOXL4 overexpression when compared with the control group (Figure 3E). Opposite changes in the percentages of G1- and G2-stage A549 cells were induced by transfection with LOXL4 siRNA (Figure 3E), indicating the promotive effect of LOXL4 expression on cell cycle progression in A549 cells. In addition, flow cytometry studies revealed the percentages of apoptotic A549 cells were substantially reduced by LOXL4 overexpression but were significantly increased by treatment with LOXL4 siRNA (Figure 3F). These results showed LOXL4 could effectively promote the proliferation and cell cycle progression of NSCLC cells and inhibit their apoptosis.
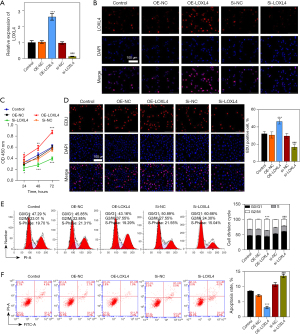
LOXL4 promoted migration, invasion, ECM accumulation, and EMT in A549 cells
We examined the influence of LOXL4 expression on A549 cell migration, invasion, ECM accumulation, and EMT progression. Our data showed the migration ability of A549 cells was significantly increased by LOXL4 overexpression, but greatly decreased by treatment with LOXL4 siRNA (Figure 4A). Additionally, Transwell assays showed the invasive ability of A549 cells was increased by LOXL4 overexpression and decreased by LOXL4 silencing (Figure 4B). Moreover, the levels of β-catenin, N-cadherin, and vimentin proteins in A549 cells were significantly increased by LOXL4 overexpression but remarkably reduced by LOXL4 siRNA (Figure 4C). On the contrary, the level of E-cadherin protein in A549 cells was decreased by LOXL4 overexpression and increased by LOXL4 silencing (Figure 4C). Furthermore, the levels of lysyl oxidase (LOX), lysyl oxidase-like 2 (LOXL2), and collagen type I alpha 1 (COL1A1) in A549 cells were significantly increased by LOXL4 overexpression and decreased by treatment with LOXL4 siRNA (Figure 4D). Finally, the levels of Fn (fibronectin) protein in A549 cells were reduced by LOXL4 overexpression and increased by LOXL4 silencing (Figure 4D). These results showed the extensive effects that LOXL4 expression has on NSCLC cell migration, invasion, ECM accumulation, and EMT progression.
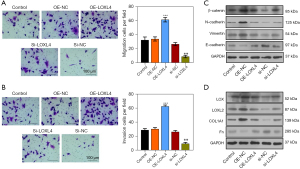
miR-183-5p regulated A549 cell proliferation, apoptosis, migration, invasion, ECM accumulation, and EMT by suppressing LOXL4 expression
To test the mediating effect of LOXL4 expression on miR-183-5p-regulated NSCLC progression, we silenced LOXL4 gene expression in A549 cells transfected with the miR-183-5p inhibitor. Our resultant data showed miR-183-5p expression in A549 cells transfected with the miR-183-5p inhibitor was greatly increased by LOXL4 overexpression (Figure 5A). The proliferation of A549 cells transfected with the miR-183-5p inhibitor was also significantly suppressed by LOXL4 siRNA when compared with negative control cells (Figure 5B,5C). Moreover, the cell cycle progression of A549 cells transfected with the miR-183-5p inhibitor was also reduced by LOXL4 siRNA (Figure 5D). On the contrary, the apoptosis rate of miR-183-5p inhibitor-transfected A549 cells was significantly increased by LOXL4 knockdown (Figure 5E), while both the migration and invasion capabilities of A549 cells transfected with the miR-183-5p inhibitor were remarkably reduced by transfection with LOXL4 siRNA (Figure 5F,5G). Importantly, the levels of β-catenin, N-cadherin, vimentin, LOX, LOXL2, and COL1A1 proteins in A549 cells transfected with the miR-183-5p inhibitor were all significantly reduced by LOXL4 siRNA (Figure 5H,5I). In contrast, the expression of E-cadherin and Fn proteins in A549 cells transfected with the miR-183-5p inhibitor was greatly increased by LOXL4 silencing (Figure 5H,5I). These results showed LOXL4 expression mediated the regulation of A549 cell proliferation, apoptosis, migration, invasion, ECM accumulation, and EMT progression via miR-183-5p.
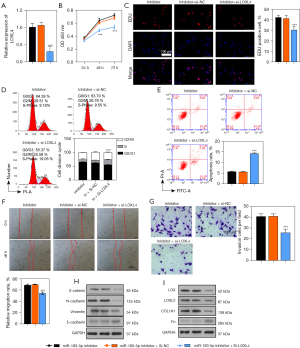
miR-183-5p enhanced the tumorigenicity of A549 cells via ECM accumulation and EMT progression
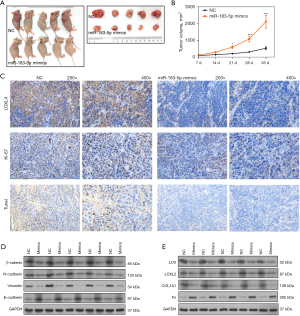
To evaluate the effects of miR-183-5p on the tumorigenicity of NSCLC cells, we injected A549 cells transfected with miR-183-5p mimics into nude mice to establish a cell line-based xenogenic model (Figure 6A). When compared with negative control mice, the size of tumors formed from A549 cells were significantly reduced by the miR-183-5p mimics (Figure 6B). A subsequent IHC assay showed the expression levels of LOXL4 and Ki-67 proteins in tumors formed by A549 cells transfected with miR-183-5p mimics were significantly lower than in the negative control groups (Figure 6C). However, the percentages of apoptotic cells in tumor tissues from the miR-183-5p mimics group were significantly higher than in the negative control groups (Figure 6C), while the levels of β-catenin, N-cadherin, vimentin, LOX, LOXL2, and COL1A1 protein expression in tumors from the miR-183-5p mimics group were much lower than in the negative controls (Figure 6D,6E). On the contrary, the E-cadherin and Fn protein levels in tumors from the miR-183-5p mimics group were much higher than in the negative control group (Figure 6D,6E). These results showed miR-183-5p could substantially promote the tumorigenicity of NSCLC cells by enhancing ECM accumulation and EMT progression.
Discussion
Epigenetic regulation of functional gene expression by microRNAs has been shown to perform critical pathogenic roles in lung cancer development (16,18). Recent investigations reported that miR-183-5p expression was significantly altered in NSCLC cells, and those alterations contributed to the development and progression of NSCLC (24,25). However, the downstream molecular events underlying the tumorigenic effect of miR-183-5p in NSCLC remain largely unknown. In the present study, we found for the first time, that decreased miR-183-5p expression had a significant negative correlation with one ECM remodeling protein (LOXL4) in clinical cancer tissues, which validates the targeting of LOXL4 expression by miR-183-5p in NSCLC cells. Overexpression or knockdown of the LOXL4 gene substantially altered proliferation, cell cycle progression, apoptosis, migration, and invasion in NSCLC cells. Importantly, we showed ECM deposition and EMT progression in NSCLC cells was greatly promoted by LOXL4 overexpression but repressed by LOXL4 knockdown. More importantly, the proliferation, cell cycle progression, apoptosis, migration, and invasion, as well as the ECM accumulation and EMT progression in NSCLC cells transfected with miR-183-5p mimics was effectively altered by LOXL4 knockdown. Finally, we validated the inhibitory effects of miR-183-5p on ECM deposition, EMT progression, and NSCLC cell tumorigenicity in a cell line-based xenograft model. Our findings provide new insights into the pathogenic mechanism of miRNA-mediated NSCLC.
The possible involvement of miRNAs in NSCLC initiation and development has attracted great attention due to the recent discovery of their important pathogenic roles (21-23). It has been extensively investigated whether miR-183-5p acts as a critical epigenetic regulator during cancer development and progression. For instance, the progression of colorectal cancer (CRC) and its resistance to radiotherapy were found to be greatly enhanced by miR-183-5p, and its effects were mediated by its targeting and suppression of Autophagy-related gene 5 (ATG5) expression (34). Moreover, the levels of miR-183-5p were recently reported to be greatly reduced in gastric cancer (GC) tissue and cell lines. The reduction in GC tissue was closely associated with its staging and metastasis, suggesting mRNA-183-5p as a potential non-coding RNA biomarker for GC progression and prognosis prediction (35). Moreover, the involvement of miR-183-5p in cancer development, progression, and treatment was also evidenced in the context of several other cancer types, such as renal cancer and bladder cancer (35,36). In this study, we clearly showed miR-183-5p was expressed at significantly lower levels in both NSCLC tissues and cell lines. We also verified the tumor suppression role of miR-183-5p in NSCLC cells by using an in vivo tumorigenicity model, and those data further supported its many functions in tumor development and progression. The tissue-specific roles and underlying mechanisms of miR-183-5p in various cancer types deserve further investigation.
The pleiotropic biological and pathogenic roles of miRNAs are known to be mainly mediated by its regulation of functional gene expression achieved via association with their promoter regions. The tumorigenesis-regulating functions of miR-183-5p were also recently shown to be realized by its ability to regulate the expression of several different genes, including eukaryotic elongation factor 2 (eEF2), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and ATG5 (34,35,37). In this study, we showed miR-183-5p could directly bind to the promoter region of the LOXL4 gene, and its overexpression or knockdown resulted in a completely opposite alteration of LOXL4 gene expression in NSCLC cells. Regarding the pathogenic roles of LOXL4, we found LOXL4 overexpression exerted positive effects on the proliferation, cell cycle progression, apoptosis, migration, and invasion capabilities of NSCLC cells, while the opposite effects were produced by LOXL4 knockdown. In Figure 3E, overexpression of LOXL4 does not lead to a higher percentage of cells in S-phase compared to the control condition. However, the percentage in G0/G1-phase and S-phase (the summation) was significantly reduced by overexpression of LOXL4. It suggested that LOXL4 promotes the transition of cells from G0/G1-phase to S-phase, or from S-phase to G2/M phase. Conversely, when we koncked the LOXL4 down, the percentage in G0/G1-phase was significantly increased and that in S-phase was significantly decreased. Therefore, it’s almost certain that the expression of LOXL4 could inluenced the transition of cells from G0/G1-phase to S-phase. More importantly, we verified the roles played by LOXL4/miR-183-5p interaction in NSCLC development by silencing LOXL4 expression in NSCLC cells transfected with an miR-183-5p inhibitor. These results convincingly proved that the effects of miR-183-5p in inhibiting NSCLC initiation and progression were mediated by its suppression of LOXL4 expression achieved by targeting the LOXL4 gene promoter region. However, a newly published study reported plasma miR-183-5p in CRC patients with lymph node metastasis was abnormally overexpressed (38). More importantly, Mo et al. (39) found miR-183-5p plays an oncogenic role in lung adenocarcinoma by targeting LATS1 and inhibiting the Hippo/YAP pathway . When taken together, these findings suggest the multiple pathogenic roles of miR-183-5p in various human cancer types and its use in tumor staging might be closely linked with its specific binding to different target genes.
LOXL4 is an lysyl oxidase enzyme possessing widespread biological and pathogenic functions due to its substantial regulation of collagen and elastin crosslinking and ECM deposition (26,27). LOXL4 has also been established as an essential mediator of cancer cell migration and metastasis because of the significant roles it plays in ECM remodeling during EMT progression (26,27). As an important component of tumor microenvironment, ECM alterations affected EMT progression. Conversely, cancer cells entering the EMT process can promote ECM alterations through multiple signal pathways. As mentioned above, LOXL4 and its isozymes (LOX and LOXL2) might synergistically modulate the accumulation and assembly of ECM components such as COL1A1 and Fn (30). However, previous studies have reported the function of LOXL4 in cancer cells to be controversial. LOXL4 was found to promote tumor development in laryngeal squamous cell carcinoma (40), liver cancer (41,42), and breast cancer (43), while Zhang (32) and Xie et. al (30) reported it may have tumor suppressive properties in lung cancer. In contrast, our current study showed LOXL4 enhanced NSCLC proliferation, migration, ECM deposition, and the EMT process. Our study proved the expression of LOX, LOXL2, COL1A1, and Fn proteins in NSCLC cells was greatly altered by overexpression or knockdown of the LOXL4 gene. Moreover, we also showed the expression of EMT biomarker proteins β-catenin, E-cadherin, N-cadherin, and vimentin was increased by LOXL4 overexpression in NSCLC cells, while expression of the epithelial marker protein E-cadherin was greatly decreased by LOXL4 overexpression. More importantly, the expression of these ECM and EMT biomarker proteins was remarkably changed in NSCLC cells co-transfected with the miR-183-5p inhibitor and LOXL4 siRNA, as well as in tumors derived from NSCLC cells transfected with miR-183-5p mimics in nude mice. Hence, we proved the pathogenic effects of the newly discovered miR-183-5p/LOXL4 interaction in NSCLC development were mediated by their regulation of ECM accumulation and EMT progression.
Conclusions
In summary, this study revealed that miR-183-5p, the expression of which was greatly repressed in NSCLC cells, serves as essential tumor suppression factor in NSCLC cells via its direct targeting of LOXL4 expression to regulate ECM deposition and EMT progression.
Acknowledgments
Funding: This research was supported by the Guangzhou Health Science and Technology Project (Grant No. 20211A011046).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-329/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-329/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-329/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-329/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All study participants signed an informed consent document giving permission for their inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved in advance by the Medical Ethical Committee of Xiangya Hospital (No. 202110993). All experimental procedures performed with mice were approved by the Experimental Animal Ethics Committee of Xiangya Medical College, Central South University (No. 202110993), in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen PH, Cai L, Huffman K, et al. Metabolic Diversity in Human Non-Small Cell Lung Cancer Cells. Mol Cell 2019;76:838-851.e5. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Mamdani H, Jalal SI. Histone Deacetylase Inhibition in Non-small Cell Lung Cancer: Hype or Hope? Front Cell Dev Biol 2020;8:582370. [Crossref] [PubMed]
- Rafei H, El-Bahesh E, Finianos A, et al. Immune-based Therapies for Non-small Cell Lung Cancer. Anticancer Res 2017;37:377-87. [Crossref] [PubMed]
- Wang X, Liu Z, Zhang L, et al. Targeting deubiquitinase USP28 for cancer therapy. Cell Death Dis 2018;9:186. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. [Crossref] [PubMed]
- Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science 2017;357:eaal2380. [Crossref] [PubMed]
- Wimalasena VK, Wang T, Sigua LH, et al. Using Chemical Epigenetics to Target Cancer. Mol Cell 2020;78:1086-95. [Crossref] [PubMed]
- O'Leary K, Shia A, Schmid P. Epigenetic Regulation of EMT in Non-Small Cell Lung Cancer. Curr Cancer Drug Targets 2018;18:89-96. [Crossref] [PubMed]
- Zang X, Gu J, Zhang J, et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis 2020;11:215. [Crossref] [PubMed]
- Zhang Y, Cheng K, Xu B, et al. Epigenetic Input Dictates the Threshold of Targeting of the Integrin-Dependent Pathway in Non-small Cell Lung Cancer. Front Cell Dev Biol 2020;8:652. [Crossref] [PubMed]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203-22. [Crossref] [PubMed]
- Legras A, Pécuchet N, Imbeaud S, et al. Epithelial-to-Mesenchymal Transition and MicroRNAs in Lung Cancer. Cancers (Basel) 2017.
- Ranković B, Zidar N, Žlajpah M, et al. Epithelial-Mesenchymal Transition-Related MicroRNAs and Their Target Genes in Colorectal Cancerogenesis. J Clin Med 2019;8:1603. [Crossref] [PubMed]
- Zou XZ, Liu T, Gong ZC, et al. MicroRNAs-mediated epithelial-mesenchymal transition in fibrotic diseases. Eur J Pharmacol 2017;796:190-206. [Crossref] [PubMed]
- Bica-Pop C, Cojocneanu-Petric R, Magdo L, et al. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell Mol Life Sci 2018;75:3539-51. [Crossref] [PubMed]
- Iqbal MA, Arora S, Prakasam G, et al. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med 2019;70:3-20. [Crossref] [PubMed]
- Duan FG, Wang MF, Cao YB, et al. MicroRNA-421 confers paclitaxel resistance by binding to the KEAP1 3'UTR and predicts poor survival in non-small cell lung cancer. Cell Death Dis 2019;10:821. [Crossref] [PubMed]
- Liu S, Li B, Xu J, et al. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front Cell Dev Biol 2020;8:213. [Crossref] [PubMed]
- Yu X, Zhang Y, Cavazos D, et al. miR-195 targets cyclin D3 and survivin to modulate the tumorigenesis of non-small cell lung cancer. Cell Death Dis 2018;9:193. [Crossref] [PubMed]
- Li C, Yin Y, Liu X, et al. Non-small cell lung cancer associated microRNA expression signature: integrated bioinformatics analysis, validation and clinical significance. Oncotarget 2017;8:24564-78. [Crossref] [PubMed]
- Wang H, Ma Z, Liu X, et al. MiR-183-5p is required for non-small cell lung cancer progression by repressing PTEN. Biomed Pharmacother 2019;111:1103-11. [Crossref] [PubMed]
- Li T, Wu C, Gao L, et al. Lysyl oxidase family members in urological tumorigenesis and fibrosis. Oncotarget 2018;9:20156-64. [Crossref] [PubMed]
- Ye M, Song Y, Pan S, et al. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol Ther 2020;215:107633. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Jena MK, Janjanam J. Role of extracellular matrix in breast cancer development: a brief update. F1000Res 2018;7:274. [Crossref] [PubMed]
- Xie S, Liu G, Huang J, et al. miR-210 promotes lung adenocarcinoma proliferation, migration, and invasion by targeting lysyl oxidase-like 4. J Cell Physiol 2019;234:14050-7. [Crossref] [PubMed]
- Peng DH, Ungewiss C, Tong P, et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene 2017;36:1925-38. [Crossref] [PubMed]
- Zhang Y, Jiang WL, Yang JY, et al. Downregulation of lysyl oxidase-like 4 LOXL4 by miR-135a-5p promotes lung cancer progression in vitro and in vivo. J Cell Physiol 2019;234:18679-87. [Crossref] [PubMed]
- Ji Y, You Y, Wu Y, et al. Overexpression of miR-328-5p influences cell growth and migration to promote NSCLC progression by targeting LOXL4. Ann Transl Med 2022;10:301. [Crossref] [PubMed]
- Zheng S, Zhong YF, Tan DM, et al. miR-183-5p enhances the radioresistance of colorectal cancer by directly targeting ATG5. J Biosci 2019;44:92. [Crossref] [PubMed]
- Li H, Pan X, Gui Y, et al. Upregulation of miR-183-5p predicts worse survival in patients with renal cell cancer after surgery. Cancer Biomark 2019;24:153-8. [Crossref] [PubMed]
- Gao JM, Huang LZ, Huang ZG, et al. Clinical value and potential pathways of miR-183-5p in bladder cancer: A study based on miRNA-seq data and bioinformatics analysis. Oncol Lett 2018;15:5056-70. [Crossref] [PubMed]
- Meng F, Zhang L. miR-183-5p functions as a tumor suppressor in lung cancer through PIK3CA inhibition. Exp Cell Res 2019;374:315-22. [Crossref] [PubMed]
- Sanjabi F, Nekouian R, Akbari A, et al. Plasma miR-183-5p in colorectal cancer patients as potential predictive lymph node metastasis marker. J Cancer Res Ther 2022;18:921-6. [Crossref] [PubMed]
- Mo J, Nie H, Zeng C, et al. Circular RNA circ_0067741 regulates the Hippo/YAP pathway to suppress lung adenocarcinoma progression by targeting microRNA-183-5p. Bioengineered 2022;13:10165-76. [Crossref] [PubMed]
- Ren P, Niu X, Zhao R, et al. Long non-coding RNA AGAP2-AS1 promotes cell proliferation and invasion through regulating miR-193a-3p/LOXL4 axis in laryngeal squamous cell carcinoma. Cell Cycle 2022;21:697-707. [Crossref] [PubMed]
- Tan HY, Wang N, Zhang C, et al. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021;73:2326-41. [Crossref] [PubMed]
- Shao J, Lu J, Zhu W, et al. Derepression of LOXL4 inhibits liver cancer growth by reactivating compromised p53. Cell Death Differ 2019;26:2237-52. [Crossref] [PubMed]
- Yin H, Wang Y, Wu Y, et al. EZH2-mediated Epigenetic Silencing of miR-29/miR-30 targets LOXL4 and contributes to Tumorigenesis, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Theranostics 2020;10:8494-512. [Crossref] [PubMed]
(English Language Editor: B. Draper)


