Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer
Introduction
The use of minimally invasive surgery (MIS), including video-assisted thoracic surgery (VATS) (1-6) and robotic lobectomy (7-16), for the treatment of early-stage non-small cell lung cancer (NSCLC) has increased rapidly. The majority of experience has been in early stage disease, and because of the benefits with respect to hospital stay, morbidity and cost have made MIS a preferred approach over thoracotomy. However, data regarding MIS for the treatment of locally advanced NSCLC, particularly following induction chemotherapy has been limited.
Recently a limited number of case series have been published on the feasibility of the VATS approach for locally advanced NSCLC (17-20). The main focus of these studies was the feasibility of a minimally invasive approach in carefully selected patients. Only one (17) looked at patients that received induction chemotherapy uniformly, although all four studies included some fraction of patients undergoing preoperative therapy. Three of the studies (17-19) did report some survival data that appeared to be consistent with historical comparisons, although only one (19) overtly compared VATS versus open groups. There are currently no published series of robotic surgery for locally advanced disease.
In our institution all three approaches are utilized to treat patients with locally advanced disease. In order to assess the feasibility and survival associated with these approaches, we compared the outcomes of patients who underwent MIS or open lobectomy for locally advanced disease. We considered true locally advanced disease to be those patients with clinical stage II-III disease who underwent induction chemotherapy.
Methods
Patient selection
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC). The study was conducted using data from a prospectively maintained database on surgical treatment of NSCLC, covering patients treated between January 2002 and December 2013 at MSKCC.
All patients included in the analysis fit the following criteria: (I) the disease was histologically defined NSCLC; (II) the disease was clinical stage II or stage IIIa by the seventh American Joint Committee on Cancer (AJCC 7) staging system (21); (III) the patient underwent lobectomy; (IV) the resection was preceded by induction chemotherapy or chemoradiotherapy.
We excluded patients with a history of concurrent malignant disease, patients with other previous primary cancers, and patients who had a lung resection procedure other than lobectomy, such as wedge resection, segmentectomy, bilobectomy, pneumonectomy, and chest wall resection. Operative death was defined as death within 30 days of the operation or any time after the operation if the patient did not leave the hospital alive.
Patients were retrospectively classified into two groups on the basis of the surgical approach: MIS group (VATS and robotic lobectomy) and thoracotomy group. Patients undergoing conversion were analyzed by an intent-to-treat analysis and remained in their original group and were not crossed over.
Surgical procedures
At our institution surgeons approached locally advanced disease by thoracotomy, VATS or robotic techniques. Each surgeon that performed MIS (VATS, robotic) conformed to the Cancer and Leukemia Group B (CALGB) 39802-consensus technique of MIS lobectomy (5). The da Vinci Surgical System (Intuitive Surgical, Mountain View, CA, USA) was used to perform robotic lobectomy with either a 3- or 4-arm approach previously described (14). VATS lobectomy was performed via a 4-cm utility incision at the anterior axillary line, at the fourth or fifth intercostal space, without rib spreading. A port at the eighth or seventh intercostal space, at the posterior axillary line, was used for camera visualization, and a posterior port was used for lung retraction and stapler insertion. Thoracotomy lobectomy was performed through a standard, partial muscle-sparing posterolateral incision. Systematic mediastinal lymph nodal dissection or sampling was performed. Conversion was defined as the use of a rib-spreading thoracotomy at any point after initiation of hilar dissection by an MIS approach.
Statistical analysis
Fisher’s exact test and the Wilcoxon rank sum test were used to compare patient and disease characteristics, as well as postoperative outcomes, between patients in the MIS and thoracotomy groups. Since the distribution of sex, smoking status, pulmonary function, clinical stage, and tumor cell differentiation were comparable between the two groups (Table 1), we did not perform propensity score matching in further analysis. Overall survival (OS) was calculated from the day of surgery to the time of death. Patients who did not die during the study period were censored at the date they were last confirmed to be alive. Disease-free survival (DFS) was calculated from the day of surgery to the date of cancer recurrence or death from any cause. Patients who did not have a recurrence or who did not die during the study period were censored at the date they were last confirmed to be alive with no evidence of disease. Both endpoints were estimated using the Kaplan-Meier method. Univariate associations between patient, disease, or treatment factors and OS and DFS were analyzed using Cox proportional hazards regression. Multivariate Cox regression models were built using factors with P<0.20 in univariate analyses. A two-sided P value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using the “survival” and “survcomp” packages in R (version 2.11.1; R Development Core Team).
Full table
Results
General patient characteristics
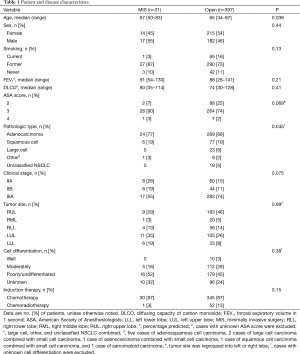
In total, 428 patients fit the criteria for inclusion in this study: 31 treated with MIS approaches (17 robotic and 14 VATS) and 397 treated with thoracotomy (Table 1). Patients in the MIS group were older than those in the thoracotomy group (P=0.038). Adenocarcinoma was the predominant pathologic type in both groups but was observed more often in the MIS group (P=0.045). The distribution of sex, smoking status, pulmonary function, ASA (American Society of Anesthesiologists) score, clinical stage, and tumor cell differentiation were comparable between the two groups.
Operation-related and postoperative outcomes
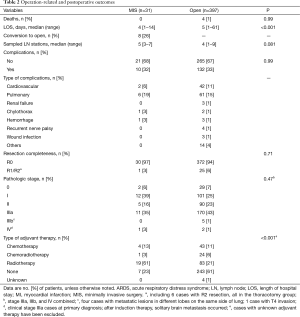
Results of the surgical treatment in all patients are seen in Table 2. Operative complications, extent of resection, and final pathologic stage were comparable between the two groups. Perioperative mortality was comparable in both groups as well, although there were no deaths in the MIS group. Eight patients were converted to thoracotomy, for various reasons: five for extent of disease, two for severe adhesions, and one for bleeding. More stations of lymph nodes were sampled in the MIS group than in the thoracotomy group, but the difference was not statistically significant (P=0.081). Patients undergoing MIS had a shorter length of hospital stay (P<0.001). A higher proportion of patients in the MIS group underwent adjuvant therapy, primarily radiotherapy (61%) (P<0.001).
Full table
Survival comparison
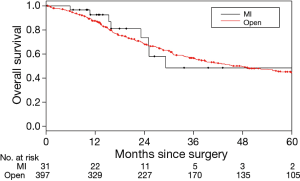
The median follow-up was 40.7 months. Tumor recurrence or death occurred in 258 cases (222 deaths, 36 alive with disease) during follow-up. The median OS was 29.2 months for the MIS group and 45.4 months for the thoracotomy group; the corresponding 3-year OS were 48.3% and 56.6%. The difference between the groups was not statistically significant (P=0.84) (Figure 1). The only variable associated with OS on univariate analysis was age: older patients had a higher risk of death (P=0.027) (Table 3). In the multivariate analysis, only age was independently associated with OS (P=0.045) whereas clinical stage, forced expiratory volume in 1 second, and surgical approach (P=0.99) were not associated with OS.
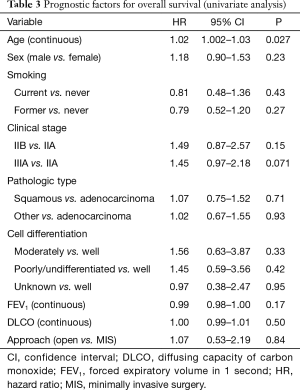
Full table
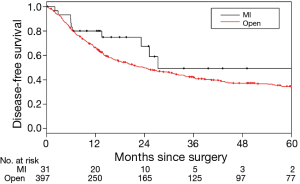
The median DFS for the MIS and thoracotomy groups were 27.3 and 23.6 months, respectively; the corresponding 3-year DFS were 49.0% and 42.1%, respectively. The difference between the groups was not statistically significant (P=0.19) (Figure 2). No factors were associated with DFS in univariate or multivariate analysis (Table 4).
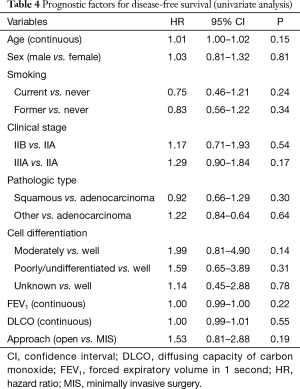
Full table
Discussion
Despite the increasing use of MIS in recent years, thoracotomy remains the most common approach for lobectomy in the United States (1-3), and the relative merits of MIS procedures for the treatment of locally advanced NSCLC in particular are unclear. For locally advanced NSCLC with established nodal metastases, multimodality therapy with induction chemotherapy is a feasible and preferred approach (22,23). However, utilization of MIS approaches is increasing, and it is therefore important to establish the role of VATS and robotics in the multimodality treatment of of patients with more advanced disease.
In this study, we compared survival and other outcomes in patients who underwent MIS compared with thoracotomy for lobectomy for NSCLC following induction chemotherapy. Our data showed that OS and DFS were comparable between the two groups, suggesting that in appropriately selected patients with locally advanced NSCLC, MIS approaches are feasible and can result in similar DFS and OS to those following thoracotomy.
Because of quicker in hospital recovery and reduced perioperative morbidity in certain patients compared with thoracotomy (1-6), MIS lobectomy has been used increasingly during the last decade, although no substantive prospective, randomized trials directly comparing the two have every been performed. It is interesting that we observed similar rate of surgical complications between approaches, perhaps due to the similar groups of patients all with locally advanced disease and good performance status. In addition, even though the only perioperative deaths were in the thoracotomy group (4/397) there was no difference in mortality. Length of hospital stay was shorter for the MIS group than for the thoracotomy group, likely due to shorter chest tube duration. However, due to the retrospective nature of the study we were lacking specific data in this regard, and this is one of the limitations of this study. These findings indicate that, with careful selection of patients, MIS approaches are safe and oncologically sound with potential benefits in hospital recovery.
In patients undergoing complete surgical resection adjuvant chemotherapy has been shown to benefit patients with pathological stage II-III disease (24,25). However, the ability for patients having a traditional thoracotomy to receive adjuvant chemotherapy has been limited. In the ANITA trial of adjuvant chemotherapy only approximately 60% of such patients were able to complete three cycles of treatment (26). Thus, at our institution even for clinical stage II disease we favor the use of induction therapy prior to surgical resection.
Long-term data on the use of MIS for locally advanced NSCLC are lacking. Hennon and coauthors from Roswell Park reported on 125 consecutive patients whom were evaluated for thoracoscopic lobectomy for advanced NSCLC (19). Eleven patients were excluded for chest wall involvement, and 19 patients had planned thoracotomy. Of the remaining 95 patients, 73 (77%) had successful MIS lobectomy. Only 19% of their patients underwent induction therapy. Like our findings, there were no differences in perioperative morbidity, mortality or survival. However, a higher proportion (37.2% vs. 5.2%) of the thoracoscopic group were able to undergo adjuvant therapy.
Huang et al. recently reported on 43 patients with NSCLC who were treated with VATS following induction therapy. They found good feasibility, good safety, and an acceptable 3-year OS (17). One patient underwent conversion, and seven patients were reported to have had a “hybrid” procedure for a total conversion rate of 19%. This is consistent with ours and other studies. Unfortunately, this study had only a single arm, and no comparison to standard thoracotomy was performed. Two other studies published recently also found good feasibility and safety for the VATS approach in patients with locally advanced NSCLC; however, most of the patients in these two studies did not receive induction therapy (18,20). Nakanishi and colleagues reported on 76 consecutive patients over a 9-year period, analyzing their results in three different time periods (18). Conversion rate was low (2.6%, 2/76), though the rate of bilobectomy (14.5%) and pneumonectomy (15.8%) were substantial. Gonzalez-Rivas and coauthors reported on 130 patients that had uniportal VATS for treatment of NSCLC (20). Forty-three patients were considered to have locally advanced disease without induction therapy. Complication rates were similar between early stage and advanced stage patients, suggesting VATS is feasible for more advanced disease. Once again pneumonectomy rate was 14%.
Our report has limitations. First, by nature of the disease and retrospective design of the study, there is considerable selection bias which may influence the comparison of outcomes between groups. Indeed, patients who underwent MIS approaches were older and tended to have lower stages of disease. The results should be interpreted as reflective of the current practice at our institution, in the context of careful selection of patients who are eligible for MIS. Definitive conclusions regarding comparisons between the two surgical approaches can be drawn only from randomized studies or larger matched case-control studies. Second, the sample size of the MIS group was small and reflects the experience of one tertiary care center. There were not enough individual VATS or robotic cases to conduct a relevant subgroup analysis to see if there are any advantages of robotics. Future multicenter studies are likely to provide more generalizable results.
In conclusion, our findings suggest that in appropriately selected patients with locally advanced NSCLC MIS approaches to lobectomy (VATS and robotic) following induction therapy are feasible and associated with comparable survival to that following thoracotomy. Additional multicenter studies are warranted to yield greater insight into the feasibility and validity of our findings.
Acknowledgements
Funding: Dr. Hao-Xian Yang was supported in part by the State Scholarship Fund of the China Scholarship Council (201306385029). This work was supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. v. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin 2014;24:157-62. vi. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5 Suppl 3:S267-73. [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. Erratum in: J Thorac Oncol 2007;2:985. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. Erratum in: Lancet Oncol 2006;7:797. [Crossref] [PubMed]