Right upper lobectomy performed as dividing posterior ascending artery-bronchus-pulmonary vessels is alternative to primary indolent scar carcinomas
Introduction
Video-assisted thoracic surgery (VATS) has been the standard treatment for early-stage non-small-cell lung cancer (NSCLC). A large proportion (37.2%) (1) of pulmonary cancers occur in the right upper lobe and are treated surgically by right upper lobectomy (RUL), which takes into account the complicated anatomical structure of the right upper lobe. In the single-direction approach of Liu et al. (2), the pulmonary vessels and then the bronchus are divided as in conventional RUL, but in both methods, problems with the hilar structures are encountered. Yan (3) summarized a reliable posterior approach, in which first the posterior ascending artery branch and then the right upper bronchus are divided, followed by the pulmonary vessels. However, in this method the surgeon stands posterior to the patient, which requires greater manipulation of the lobe, increasing the risk of vessel injuries. Since 2010, we have used VATS to perform RUL according to a technique that we refer to as posterior single-direction aBVA, in which first the posterior ascending artery branch is divided, followed by the bronchus and the pulmonary vessels.
Several different strategies for treating small nodules in the lungs have been described. Veronesi et al. (4) performed a cohort study evaluating the volume-doubling time (VDT) according to changes in the size and solid components of the nodules on sequential low-dose computed tomography (CT) screening. Slow-growing (VDT, 400–599 days) and indolent (VDT ≥600 days) tumors comprised approximately 25% of incident cases. Many of these may have been over-diagnosed and should have been dealt with by minimally invasive limited resection or even nonsurgical treatment. Indolent pulmonary cancers comprise ground glass opacities (GGO), solid and cystic nodules, the pathological morphology of which is usually a lepidic pattern, as well as carcinoids and scar adenocarcinomas. Farooqi et al. (5) reported a case of a 56-year-old male smoker who had a nodule with a cystic airspace and thin wall in the right lower lobe. The patient was followed up for 33 months, at which time the CT scan showed enlarged solid components and a thickened cyst wall. The pathological diagnosis, based on surgical tumor tissues, confirmed adenocarcinoma surrounding the entire cystic airspace. Their study thus warned of the possible development of a pulmonary malignancy subsequent to the detection of a solitary cystic airspace with increasing wall thickness. Here we describe a patient who, after implementing a “wait and see” approach, was diagnosed with pulmonary indolent scar carcinoma that was then surgically treated.
Clinical data
A 42-year-old male non-smoker was referred to Guangdong General Hospital (China) due to a lesion in the right upper lobe detected during a routine physical examination. The first positron emission tomography/CT (PET/CT) scan, performed in November 2011, showed a solitary cavitary lesion in the right upper lobe. During regular follow-up for 3.5 years, no radiological signs of malignancy were identified until June 2015 when the solid components in the wall of the carcinomatous cavity had enlarged (2.1 cm, SUV max =1.7), but without any local or distant metastases. Suspicion of indolent scar carcinoma led to the decision of RUL via VATS. The preoperative tumor stage was clinical T1bN0M0, stage Ia.
Operative techniques
The patient received double-lumen endotracheal tube intubation for unilateral lung ventilation under general anesthesia and was placed in the standard left lateral decubitus position. Specific details of this surgical procedure are provided in (Figure 1).

One access incision (3–4 cm) was made along the anterior axillary line in the fourth or fifth intercostal space (ICS), with an additional camera port (1–1.5 cm) located on the posterior axillary line one ICS lower than the access incision (Figure 2) and without rib spreading. A plastic wound protector was applied to prevent implantation metastasis, and an additional suction tube was fixed to maintain a clear surgical field. The procedure was facilitated by the use of a high-definition 30° thoracoscope, which broadened the horizons through the camera port. An electrocoagulation hook designed by Xue-Ning Yang was used in a sharp dissection of the anatomical structures. Other instruments included a powered Echelon stapler, a curved suction device, double-jointed forceps, lymphatic node forceps, and an endoscopic grasper for grasping small gauze pads.
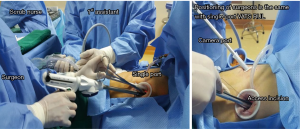
The sole surgical assistant stood on the dorsal side of the patient, holding the thoracoscope. The surgeon stood on the ventral side, holding the curved suction in his left hand to assist exposure and dissociation by the electrocoagulation hook or endoscopic stapler which was held in his right hand.
The surgery began with exploration of the lesion located in the right upper lobe and exclusion of intrathoracic dissemination. An extended wedge resection was performed with a surgical margin of 2 cm from the lesion. After sufficient tumor tissues had been obtained for pathological diagnosis, additional tumor tissues, paraneoplastic and normal lung tissues were resected for storage in our tumor specimen bank for subsequent analysis of their gene profiles.
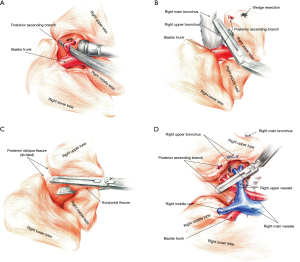
After the visceral pleura at the crossover of the horizontal and posterior oblique fissure was dissected, the interlobar lymph nodes at that site were explored. These nodes are usually regarded as a landmark to avoid injuries to the posterior ascending branch of the right upper artery. While pushing the right upper lobe to the inferior side with the curved suction, the surgeon used the electrocoagulation hook to open the superior mediastinal pleura, to identify the apico-anterior arterial trunk and dissociate the superior hilar (station 10) lymph nodes. The posterior mediastinal pleura was then opened up to the point of the oblique fissure, to expose the wedge of the right upper bronchus. A blunt dissection was performed using curved forceps, followed by manual establishment of a tunnel running through the oblique fissure back to the posterior hilum. Facilitated by the traction of a silk thread, an ENDO-GIA45/3.5 cm stapler was inserted into the tunnel, and the posterior oblique fissure was dissected. The right upper lobe was displaced superiorly and anteriorly, allowing dissociation of the station 12 lymph nodes. The anterior mediastinal pleura was opened between the right upper and middle pulmonary vein by the electrocoagulation hook. Another manual tunnel was made through the anterior to posterior hilum, facilitating the division of the fused horizontal fissure using an EGIA60AXT stapler.
Once the pathological results of the intraoperative frozen section confirmed pulmonary invasive adenocarcinoma, an ENDO-GIA30/2.5 cm stapler was used to divide the posterior ascending branch of the right upper artery (a in the aBVA) by displacing the right upper lobe superiorly and anteriorly to further expose the artery. The posterior mediastinal pleura was opened to the edge of the right upper bronchus. An ENDO-GIA45/4.8 cm stapler was used to close and then dissect the right upper bronchus (B in aBVA) once the well-inflated right middle and lower lobes were identified when bilateral lung ventilation was performed by the anesthetist. Afterwards, the remaining adhesions around the hilum were freed as much as possible. The lobar and hilar lymph nodes should be dissociated to the distal end of the pulmonary vessels or just divided to minimize the thickness of residual hilar structures. An EGIA45AVM stapler was then used to divide the remaining branches of the pulmonary arteries and veins simultaneously (VA in aBVA) (Figure 3).
Next, the right upper lobe was removed using a specimen bag to minimize contact with the incision and to eliminate the possibility of implantation metastases. The N1 and N2 lymph nodes were removed using systematic node dissection based on standard principles. Warm normal saline was used for thorough washing, and a chest tube was inserted through the camera port, as in conventional procedures.
Pathological diagnosis based on the paraffin-embedded tumor tissues confirmed pulmonary invasive adenocarcinoma with a combined acinar (80%) and papillary (20%) growth pattern [pT1bN0M0, stage Ia; World Health Organization Classification, 2015, (7)]. Gene profiles showed that the tumor was negative for epidermal growth factor receptor (EGFR) and ALK expression.
Comments
Our alternative RUL procedure using VATS called the posterior-single aBVA approach, whose general principle is to proceed from the central to the peripheral hilar anatomical structures (Figure 3). The visceral pleura at the crossover of the oblique and horizontal fissures is opened first, to identify and subsequently dissect the posterior ascending branch of the right upper artery (a in the aBVA). The oblique fissure is then divided for better exposure of the right upper bronchus, which in patients with totally fused fissures should be divided last. An electrocoagulation hook is used to open the mediastinal pleura posteriorly, superiorly, and anteriorly, accompanied by dissociation of the hilar lymph nodes and identification of the pulmonary vessels. The right upper bronchus (B in aBVA) is then dissected using a stapler. Subsequent division of the horizontal fissure is facilitated by the manual establishment of a tunnel; incomplete fissures should be divided during the last step. Residual hilar structures are dissociated as much as possible, either sweeping the hilar and lobar lymph nodes to the distal end of the lobe or dissociating them. A stapler is used to dissect the remaining pulmonary arteries and veins (VA in aBVA).
To some extent, the efficient use of specific instruments optimized our surgical procedure. The electrocoagulation hook designed by Xuening Yang has a small obtuse angled hook (145° from the longitude axis) at its apical end. With the electrocoagulation hook’s combined function of cutting and coagulating, the surgeon can carry out sharp, subtle dissections and quick hemostasis during the operation. Moreover, the electrocoagulation hook is sufficiently long with high plasticity, which aids in overcoming surgical difficulties at anatomic sites of high angularity.
Our alternative aBVA procedure has been performed using a double- or single-port (Figure 2) in hundreds of patients. Statistical analyses have confirmed its advantages compared with conventional surgical methods, including: (I) a shorter average operation time and less blood loss (8); (II) lower risks of conversion to open thoracotomy, and more evidence of the reliability, feasibility and safety of the procedure; (III) shorter intubation duration and postoperative hospitalization, promoting fast postoperative recovery; and (IV) fewer postoperative complications. The potential difficulties of aBVA are (I) the identification of the posterior ascending branch of the right upper artery; and (II) the division of fused fissures through a manual channel. In addition, the procedure demands the surgeon’s familiarity with hilar anatomical structures.
In summary, we demonstrated the technical feasibility, safety and effective application of the aBVA, a reliable and reproducible VATS RUL procedure. Its effectiveness will be validated as it is used to treat additional candidate patients with right upper lobe carcinomas requiring RUL.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81001031 and 81372285); the grant S2013010016354 from the Natural Science Foundation of Guangdong; Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006); Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (Grant No. 201402031); Research Fund from Guangzhou Science and Technology Bureau (Grant No. 2011Y2-00014).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Yan TD. Surgical atlas of thoracoscopic lobectomy and segmentectomy. Ann Cardiothorac Surg 2014;3:183-91. [PubMed]
- Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. [Crossref] [PubMed]
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Zhai HR, Nie Q, Dong S, et al. A novel procedure of right upper lobectomy as dividing posterior ascending artery-bronchus-pulmonary vessels for primary indolent scar carcinomas. Asvide 2016;3:222. Available online: http://www.asvide.com/articles/981
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. [Crossref] [PubMed]


