
Efficacy and safety of colistin sulfate in the treatment of infections caused by carbapenem-resistant organisms: a multicenter retrospective cohort study
Highlight box
Key findings
• The rate of clinical improvement in patients receiving the colistin sulfate in the treatment of carbapenem-resistant organism (CRO) infections was 70.5%, and 4.1% patients experienced acute kidney injury during the treatment.
What is known and what is new?
• Polymyxins are polypeptide antibiotics that mainly include polymyxin B (PMB), colistin sulfate, and colistimethate sodium (CMS). Several studies have confirmed the clinical efficacy and safety of polymyxin B and CMS in the treatment of CRO infections.
• Colistin sulfate has favorable clinical improvement rate against different pathogenic bacteria and causes less adverse reactions than previously reported.
What is the implication, and what should change now?
• Colistin sulfate is a reasonable choice for the treatment of CRO infections. However, the possible kidney injury caused by the drugs requires intensive monitoring. We intend to conduct a randomized controlled trial (RCT) to extend understanding of drugs such as colistin sulfate in the future.
Introduction
Recently, the bacterial resistance rate has continued to increase year by year, and antimicrobial resistance has become a global crisis threatening human health (1). Carbapenem-resistant organisms (CRO) cause the most severe infections (2). CROs mainly include carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Pseudomonas aeruginosa (CRPA), and carbapenem-resistant Acinetobacter baumannii (CRAB) (3). Among the CROs, CRPA and CRAB are the most prevalent worldwide, followed by Enterobacteriaceae members, including carbapenem-resistant Klebsiella pneumoniae (CRKP) and Escherichia coli (CRECO) (4). The incidence of CRO varies worldwide depending on the region and the organism (5). These resistant bacteria are often accompanied by high morbidity and mortality (6), and pose great challenges for clinical anti-infective therapies.
The current effective agents for CROs include tigecycline (TGC), ceftazidime/avibactam and polymyxin (7). Although, some new drugs have successively applied for market approval; however, clinically accessible drugs remain limited in China (8). Given the limited efficacy of the currently available antimicrobial agents and the lack of new antimicrobial agents, polymyxins have become an important treatment option for CRO infections (9). The results of the CHINET China antimicrobial surveillance study in 2021 showed that polymyxins are highly sensitive to CRKP, CRPA, and CRAB (10). In addition, polymyxins have been recommended as an important treatment for CRO infections (11).
Polymyxins are polypeptide antibiotics, and mainly include polymyxin B (PMB), colistin sulfate, and colistimethate sodium (CMS). Currently, several studies have investigated the clinical efficacy and safety of polymyxin B and CMS in the treatment of CRO infections, including CRPA, CRAB, and CRKP infections (12-15). The colistin sulfate is available late. However, there is lack of data on efficacy, adverse events for colistin sulfate in the treatment of CRO infections. Thus, this study sought to investigate the rate of clinical improvement and adverse reactions of colistin sulfate in the treatment of severe infections caused by CRO in critically ill patients and assess the factors associated with 28-day all-cause mortality. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-336/rc).
Methods
Study design and patients
This multicenter retrospective cohort study included patients who received colistin sulfate due to CRO infections during July 2021 and May 2022. Patients were included in this study if they received intravenous colistin sulfate for at least 48 hours during hospitalization from July 2021 to May 2022, were aged ≥18 years, had microbiological evidence and susceptibility results showing CRO infections, or had been empirically applicated for colistin sulfate without microbiological evidence. Patients were excluded from this study if they had received other polymyxins within 7 days before their inclusion in the study. The study protocol was approved by the Research Ethics Committee of Henan Provincial People’s Hospital [(2021) Ethical Review New Technology (No. 86)] and was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013), Good Clinical Practice, and the applicable regulatory requirements. The other 10 hospitals were informed and agreed with the study. Informed consent was not required, as no intervention was performed and no information identifying any patient was included.
Data
The clinical data of the patients were collected by searching the electronic medical records of the hospitals. The following information was collected: age, gender, past medical history, diagnosis at ICU admission, site of infection, pathogen, vital signs, acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score, biochemical parameters, combined antibacterial agents, ICU stay, dose and duration of colistin sulfate therapy, renal function, clinical and microbiological findings, adverse reactions to colistin sulfate, and 28-day all-cause mortality. The severity of the underlying disease was assessed according to the APACHE II score and SOFA score on the day before enrollment.
Microorganisms and definitions
The culture, identification, and susceptibility testing were performed at each participating site. The polymyxin susceptibility testing was performed using an international- standard broth microdilution. According to the European Committee for Antimicrobial Susceptibility Testing, isolates with a minimum inhibitory concentration (MIC) ≤2 mg/L were considered susceptible to polymyxin (16). Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection; for clinical operationalization, organ dysfunction was indicated by an increase in the sepsis-related SOFA score of ≥2 points. Patients who had been clinically defined to have septic shock required vasopressors to maintain mean arterial pressure ≥65 mmHg and a serum lactate level ≥2 mmol/L in the absence of hypovolemia (17).
Dosing regimen of colistin sulfate
The optimal dose of colistin sulfate (Shanghai Pharma New Asia Pharmaceutical Co. Ltd., Batch number: 110001) was recommended in the package insert, and the intravenous dose of colistin sulfate in adults was 1–1.5 MU/d in 2–3 divided intravenous drips, with a maximum daily dose of no more than 1.5 MU.
Outcome assessment
Clinical response was assessed jointly by the attending physician and pharmacist as per the clinical and microbiological criteria after colistin sulfate withdrawal. The clinical outcomes were divided as follows: clinical cure (the resolution of symptoms/signs and antibiotics-free); clinical remission (the partial resolution of symptoms/signs but not antibiotics-free); and clinical failure (the persistence or progression of symptoms/signs, and/or the development of new symptoms/signs suggestive of new infection). The definition of clinical improvement included “clinical cure” and “clinical remission”. The microbial responses included clearance (no pathogen was found in the infected site after bacterial culture) and non-clearance (the number of bacteria was not reduced or was reduced but not completely cleared). The microbiologic response was assessed as the eradication of the original causative organism from the subsequent cultures by day 3 of therapy. In the absence of follow-up cultures, the eradication of the causative organism was assessed based on the patient’s temperature, inflammatory indicators, signs, and symptoms etc.
An adverse event was defined as a reaction that was clearly harmful or uncomfortable due to the use of colistin sulfate. A common adverse event was nephrotoxicity. As per the Kidney Disease Improving Global Outcomes criteria, nephrotoxicity was defined as one of the following: (I) an increase in serum creatinine (Scr) of >26.5 µmol/L (0.3 mg/dL) within 48 hours; (II) an increase in Scr of >1.5-fold above the baseline level within 7 days; and (III) urine output <0.5 mL/(kg·h) that persists for >6 hours (18). Neurotoxicity was defined as dizziness, facial or peripheral paresthesia, vertigo, visual impairment, hallucinations, mental confusion, ataxia, neuromuscular block, or seizures during colistin treatment.
Statistical methods
SPSS 26.0 software was used for the statistical analysis of the data. The normally distributed quantitative data are expressed as the mean ± standard deviation (x±s), and the independent sample t-test was used for comparisons between two groups. The non-normally distributed quantitative data are presented as the median (quartiles) [M (Q1, Q3)], and the Mann-Whitney U test was used for comparisons between two groups. The qualitative data are described as the number and percentage, and the χ2 test or the Fisher’s exact test was used for comparisons between groups. A Cox regression survival analysis was conducted to assess the independent predictors of 28-day all-cause mortality for colistin sulfate. A P value <0.05 was considered statistically significant.
Results
Demographic data
The data of 122 patients with CRO infections treated from July 2021 to May 2022 were included in the analysis cohort (Figure 1). The baseline demographics, clinical characteristics, and outcomes of the patients are presented in Table 1. A high proportion of the patients had concomitant diseases at admission, most commonly cardiovascular disease, diabetes, and chronic neurological disease. All the patients meet the criteria for sepsis before the initiation of the colistin sulfate therapy, and 25.4% (n=31) of the patients had septic shock. The pathogen culture results were positive for 117 (96%) patients, and the highest proportion of isolates was Acinetobacter baumannii (AB) in 41 patients (33.6%). Of all the patients, 91 (74.6%) developed a single-site infection, of which pulmonary infection (50.8%) was the most common site of infection. 9.8% patients were treated with monotherapy , the remaining patients received combination therapy. Colistin sulfate combined with carbapenems (n=50, 41%) was the most common combination regimen.
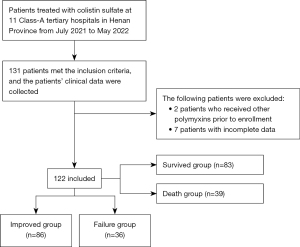
Table 1
| Variable | Value |
|---|---|
| Age (years) | 62 [54, 75.25] |
| Gender (male) | 93 (76.2) |
| Pre-existing disease | |
| Cardiac disorder | 69 (56.6) |
| Diabetes | 38 (31.1) |
| Hypertension | 7 (5.7) |
| Nervous system disorders | 26 (21.3) |
| Chronic lung disease | 19 (15.6) |
| Liver disease | 7 (5.7) |
| Malignancy | 6 (4.9) |
| Kidney disease | 10 (8.2) |
| Immunosuppressant status | 2 (1.6) |
| APACHE II | 18.42±7.34 |
| SOFA score | 8 [5, 10] |
| Sepsis | |
| Non-shock | 91 (74.6) |
| Shock | 31 (25.4) |
| Life-sustaining therapy | |
| Mechanical ventilation | 88 (72.1) |
| ECMO | 21 (17.2) |
| CRRT | 38 (31.1) |
| Infected pathogen | |
| Acinetobacter baumannii | 41 (33.6) |
| Klebsiella pneumoniae | 30 (24.6) |
| Pseudomonas aeruginosa | 7 (5.7) |
| Other | 4 (3.2) |
| Not identified | 5 (4.1) |
| Site of infection | |
| Pulmonary infection | 62 (50.8) |
| Bloodstream infection | 23 (18.8) |
| Other | 6 (4.9) |
| Multiple sites | 31 (25.4) |
| Dose and duration | |
| 1 MU/day | 93 (76.2) |
| 1.5 MU/day | 29 (23.8) |
| Course (days) | 10 [6, 14] |
| Dosing regimen | |
| Monotherapy | 12 (9.8) |
| Combined therapy | 110 (90.2) |
| Colistin sulfate + Carbapenems | 50 (41.0) |
| Colistin sulfate + Tigecycline | 14 (11.5) |
| Colistin sulfate + Ceftazidime avibactam | 3 (2.5) |
| Microbial clearance | 61 (50.0) |
| ICU stay (days) | 20 [14, 28] |
| Adverse reaction (acute kidney injury) | 5 (4.1) |
The categorical variables are presented as number (%), and the continuous variables are presented as mean ± standard deviation or median [IQR]. APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; ICU, intensive care unit; IQR, interquartile range.
Clinical and microbial reactions
Of the patients, 86 (70.5%) showed a favorable clinical response. The comparison of the improvement group and failure group showed that (Table 2) the median SOFA score was higher in the failure group than the improvement group {9.5 [7, 11] vs. 7 [4, 9], P=0.002}, the proportion of patients receiving ECMO was higher in the failure group than the improvement group (27.8% vs. 12.8%, P=0.046), and the median duration of treatment was longer in the improvement group than the failure group {12 [8, 15] vs. 5.5 [4, 9.75], P<0.001}. The shorter duration of treatment in the failure group was due to the fact that most patients chose automatic discharge. We also found a trend towards a higher clinical failure rate in patients receiving mechanical ventilation, but there was no statistical difference between the improvement group and failure group (31.8% vs. 23.5%, P=0.368). No significant difference was observed in the clinical outcomes of the septic shock and septic non-shock patients (25.8% vs. 30.8%, P=0.601). Patients receiving continuous renal replacement therapy (CRRT) had a higher clinical failure rate than those not receiving CRRT (36.8% vs. 35.5%, P=0.350). As Tables 3,4 show, there were no significant differences in the clinical outcomes of colistin sulfate in terms of the treatment of different pathogenic microorganisms and the clearance of infections at different sites.
Table 2
| Variable | Improvement group (n=86) | Failure group (n=36) | P value |
|---|---|---|---|
| Age (years) | 62 [50, 73.75] | 63.5 [57.25, 75.75] | 0.147 |
| Gender (male) | 65 (75.5) | 28 (77.7) | 0.795 |
| Pre-existing disease | |||
| Cardiac disorder | 50 (58.1) | 19 (52.8) | 0.586 |
| Diabetes | 28 (32.6) | 10 (27.8) | 0.603 |
| Nervous system disorders | 20 (23.3) | 6 (16.7) | 0.418 |
| Chronic lung disease | 13 (15.1) | 6 (16.7) | 0.829 |
| Malignancy | 6 (7.0) | 2 (5.6) | 1.000 |
| Kidney disease | 6 (7.0) | 4 (11.1) | 0.691 |
| APACHE II score | 17.87±6.92 | 19.75±8.22 | 0.199 |
| SOFA score | 7 [4, 9] | 9.5 [7, 11] | 0.002 |
| Sepsis | |||
| Non-shock | 63 (73.3) | 28 (77.8) | 0.601 |
| Shock | 23 (26.7) | 8 (22.2) | |
| Life-sustaining therapy | |||
| Mechanical ventilation | 60 (69.8) | 28 (77.8) | 0.368 |
| ECMO | 11 (12.8) | 10 (27.8) | 0.046 |
| CRRT | 24 (27.9) | 14 (38.9) | 0.232 |
| Dose and course of colistin sulfate | |||
| 1 MU/day | 65 (75.6) | 28 (77.8) | 0.795 |
| 1.5 MU/day | 21 (24.4) | 8 (22.2) | |
| Course (days) | 12 [8, 15] | 5.5 [4, 9.75] | <0.001 |
| Treatment regimen | |||
| Monotherapy | 9 (10.5) | 3 (8.3) | 0.978 |
| Combined therapy | 77 (89.5) | 33 (91.7) |
The categorical variables are presented as number (%), and the continuous variables are presented as mean ± standard deviation or median [IQR]. APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; IQR, interquartile range.
Table 3
| Variable | Improvement group (n=86) | Failure group (n=36) | P value |
|---|---|---|---|
| Infected pathogen | |||
| AB | 30 (34.9) | 11 (30.6) | 0.644 |
| KP | 19 (22.1) | 11 (30.6) | 0.322 |
| PA | 5 (5.8) | 2 (5.6) | 1.000a |
| Infected with multiple pathogens | |||
| KP + PA | 7 (8.1) | 2 (5.6) | 0.906 |
| KP + AB | 11 (12.8) | 3 (8.3) | 0.694 |
| AB + PA | 4 (4.7) | 2 (5.6) | 1.000a |
Categorical variables are presented as numbers (%). a, Fisher’s exact test was used. AB, Acinetobacter baumannii; KP, Klebsiella pneumoniae; PA, Pseudomonas aeruginosa.
Table 4
| Variable | Improvement group (n=86) | Failure group (n=36) | P value |
|---|---|---|---|
| Site of infection | |||
| Pulmonary infection | 40 (46.5) | 22 (61.1) | 0.141 |
| Bloodstream infection | 18 (20.9) | 5 (13.9) | 0.364 |
| Others | 5 (5.8) | 1 (2.8) | 0.669a |
| Multi-site infection | 23 (26.7) | 8 (25.0) | 0.601 |
Categorical variables are presented as numbers (%). a, Fisher’s exact test was used.
Adverse reactions
A total of 5 patients (4.1%) suffered from acute kidney injury during the treatment, 3 of whom had a pre-existing renal insufficiency. The mean time to peak Scr in patients with renal impairment was 6.4 days. Of the 5 patients with renal impairment, 1 had mild renal impairment, and the other 4 had moderate to severe renal impairment. The patients with renal impairment showed significantly increased creatinine than one day before enrollment but this decreased after discontinuation colistin sulfate treatment. The Scr level tended to be 1.5 times that of the baseline level in acute kidney injury patients after the use of colistin sulfate. As most of the included patients required a large amount of sedatives, it was difficult to assess the effects of colistin sulfate on their neurological status.
Independent factors associated with mortality
In the improvement group, 3 patients abandoned treatment for financial reason and died. Thus, the 28-day all-cause mortality rate was 32% (39/122). Table 5 shows the results of the Cox regression survival analysis for 28-day all-cause mortality in the CRO-infected patients treated with colistin sulfate, including their age, SOFA score, duration of treatment, and ECMO treatment. The results showed that SOFA score [hazards ratio (HR) =1. 198, 95% confidence interval (CI): 1.078–1.330, P=0.001], duration of treatment (HR =0.736, 95% CI: 0.653–0.830, P<0.001), and ECMO treatment (HR =2.373, 95% CI: 1.095–5.141, P=0.029) were significantly associated with 28-day all-cause mortality.
Table 5
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| SOFA score | 1.198 | 1.078–1.330 | 0.001 |
| Duration of treatment | 0.736 | 0.653–0.830 | <0.001 |
| ECMO treatment | 2.373 | 1.095–5.141 | 0.029 |
CRO, carbapenem-resistant organism; SOFA, sequential organ failure assessment; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
Bacterial resistance has become a great challenge in the global public health field, and CROs cause the most severe infections. The ICU is a special ward, and the presence of other pre-existing diseases, a poor immune capacity, and long hospital stays increase the probability of infection. Various invasive procedures also increase the risk of bacterial infection. The high detection rate of CROs and the current limited availability of drugs have led to the re-application of polymyxins in clinical practice. In this study, we retrospectively analyzed patients treated with colistin sulfate in the ICUs of 11 Class-A tertiary hospitals in Henan Province and included a total of 122 patients with CRO infections. The results showed that the most common infections in these patients were pulmonary and/or bloodstream infections caused by CRAB, CRKP, and CRPA. Colistin sulfate showed strong antibacterial activity against CRAB, CRKP, and CRPA, and was indicated for the treatment of infections at different sites. A good clinical response was observed in 86 patients (70.5%), who showed significant improvements in their laboratory tests, and 61 of the 122 patients (50%) achieved microbiological clearance. Thus, colistin sulfate is a reasonable choice for the treatment of CRO infections in the current treatment options are limited.
As stated above, microbial clearance was observed in 50% of the patients in this study, and the microbial outcome was independently associated with the APACHE II score for the severity of the pre-existing disease (20.42±7.88 vs. 16.43±6.20, P=0.002) and the SOFA score (9 vs. 7, P=0.012). This finding is similar to that of Giulia study (19), who reported that a higher baseline level was significantly associated with microbial failure. We also found that microbial clearance increased significantly with the duration of the colistin sulfate treatment course (67.8% and 5.7% for ≥7 and <7 days, respectively, P<0.001). A long-course of antibiotics can treat bacteria effectively and reduce the recurrence of inflammatory infections. However, such doses may also precipitate the occurrence of bacterial resistance. In this study, only 10% of the patients received colistin sulfate alone. The clinical improvement rate (P=0.978) and bacterial clearance (P=0.563) did not differ significantly between the colistin sulfate monotherapy and colistin sulfate based combination therapy groups. The need for combination therapy CRO infections has been controversial. An open-label randomized controlled trial in Israel examining clinical failure showed that colistin sulfate monotherapy (156/198, 79%) did not differ significantly from the combination therapy of colistin sulfate plus meropenem (152/208, 73%) in the treatment of severe infections caused by CROs (20). In a tertiary teaching hospital study in China, Tian et al. found that 95 clinical isolates of CRKP had a high rate of heterogeneous resistance to PMB and TGC, and the resistant flora could survive under the pressure of TGC or PMB, but the combination of PMB and TGC killed some of the flora (21). In addition, 2 other studies on the use of colistin sulfate in treating CRAB found that combination therapy was synergistic, resulting in higher bacterial clearance at lower concentrations (22,23). However, as all of these studies compared different antibiotic regimens in different patient populations, the results are difficult to consolidate.
Our study differed to the above-mentioned studies in several ways. First, it was an observational study, and the concomitant antibiotics were determined by the attending physician based on the type and severity of the infection. Thus, the combination regimens were unlimited, which more closely reflects clinical practice. Second, this was a clinical study, and clinical outcomes can differ to those of in vitro experiments. Thus, the clinical improvement rate did not differ significantly between the colistin sulfate monotherapy and combination therapy groups may be due to the different disease severity of the patients and the resistance mechanism of pathogenic bacteria. In practice, antibiotic regimens are determined according to the individual situation of the patient, including the disease severity, pathogenic microorganisms, and mechanisms of resistance.
The main adverse reaction caused by polymyxins is nephrotoxicity. Adverse reactions occurred in 5 (4%) patients in this study, who all suffered from kidney injury. The etiology of acute kidney injury is complex and varied, and may be related to many factors. In these 5 patients, the kidney injury resulted from not also being administered other nephrotoxic agents and was temporally associated with the use of colistin sulfate. Thus, renal injury may be caused by colistin sulfate. Creatinine levels gradually decreased in 3 patients after discontinuation colistin sulfate treatment, and the monitoring of renal function was not continued in the other 2 patients due to their abandonment of the treatment. Polymyxin antibiotics are currently reported to have a slightly lower incidence of nephrotoxicity than previously. Falagas et al. observed the incidence of nephrotoxicity in patients who underwent long-term intravenous colistin sulfate treatment and included patients who received intravenous colistin sulfate for >4 weeks to treat multidrug-resistant organism infections, and showed that median creatinine value increased by 0.25 mg/dL during the treatment compared to the baseline value (P<0.001), but had almost returned to the baseline value at the end of the treatment (P=0.67). No serious toxicity was observed in the group of patients who received a long-term intravenous colistin sulfate treatment (24). Xia et al. found that approximately 10% of patients treated with polymyxin B for CRO infections developed acute kidney injury, but 23.5% recovered after discontinuation colistin sulfate treatment (14). However, a safety study of PMB in Chinese patients, excluding those receiving renal replacement therapy, found that 38.7% developed nephrotoxicity, and the daily dose of PMB was a risk factor for nephrotoxicity (P=0.026) (25). A recent real-world study on the use of polymyxin B in the treatment of CRO infections reported that 7% of the patients developed nephrotoxicity (26).
Nephrotoxicity was low in this study, but this may have been due to the lower daily dose of colistin sulfate. In this study, 76.2% of the patients received a colistin sulfate dose of 1 MU/day. In addition, the close monitoring of renal function by clinicians reduced the incidence of nephrotoxicity. An analysis of CMS and polymyxin B treatment-related nephrotoxicity showed that age, daily dose, and the duration of treatment were independent risk factors for the development of nephrotoxicity (27,28). However, this finding was not observed in this study. The intensive monitoring of possible nephrotoxicity caused by drugs, the early detection of polymyxin-induced renal impairment, and the timely implementation of appropriate measures should be performed to avoid further damage to renal function.
This study showed that the SOFA score (HR =1.198, 95% CI: 1.078–1.330) and ECMO treatment (HR =2.373, 95% CI: 1.095–5.141) were independently associated with increased 28-day all-cause mortality, while the treatment course of colistin sulfate was associated with a lower risk of this outcome (HR =0.736, 95% CI: 0.653–0.830). Similarly, another study on the use of polymyxin B therapy to treat pan-drug resistant AB or PA infections showed that the severity of the pre-existing disease APACHE II score was associated with increased patient mortality (29). Cai conducted a retrospective cohort study and found that the APACHE II score (adjusted odds ratio =1.14; 95% CI: 1.07–1.21) was also independently associated with an increased risk of infection-related mortality (30). The severity of the pre-existing disease SOFA score independently predicted 28-day all-cause mortality, which may partially explain the similar association with clinical failure. A sufficient duration of treatment reduces the 28-day all -cause mortality of CRO-infected patients treated with colistin sulfate, and the duration of colistin sulfate treatment was not observed to be associated with the development of kidney injury in this study.
The study had some limitations. First, this study was a retrospective cohort study with limited enrolled patients and a small sample size, which affected the power of the test and may have led to the occurrence of type-II errors in the results. Second, there were many censored data in the follow-up data of this study, which may have had a certain effect on the results. Third, the dosing regimen of colistin sulfate was not standardized and differed at the discretion of the attending physician. Fourth, because the patients had different pre-existing diseases, drug-drug interactions may have affected the clinical efficacy of and patients’ adverse reactions to the colistin sulfate.
Conclusions
CRO infections represent a significant threat to public health, and polymyxins are increasingly being used to treat such infections. This appears to be the first multicenter retrospective study to investigate the rate of clinical improvement and adverse events of colistin sulfate in treating CRO infections in China. This retrospective clinical study collected the data of ICU patients, most of whom had severe infections caused by CRO, and the results showed that colistin sulfate had favorable clinical improvement rate against different pathogenic bacteria and less adverse reactions than previously reported. Colistin sulfate is a reasonable and safe treatment option for CRO infections if the current treatment options are limited. We intend to conduct a randomized controlled trial to extend our understanding of drugs such as colistin sulfate in the future.
Acknowledgments
We thank all the hospitals that participated in this study. We thank Jingge Zhao from Henan People’s Hospital for her help in the statistical analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-336/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-336/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-336/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-336/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Research Ethics Committee of Henan Provincial People’s Hospital [(2021) Ethical Review New Technology (No. 86)] and was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013), Good Clinical Practice, and the applicable regulatory requirements. The other 10 hospitals were informed and agreed with the study. Informed consent was not required, as no intervention was performed and no information identifying any patient was included.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tängdén T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med 2015;277:501-12. [Crossref] [PubMed]
- Livermore DM, Nicolau DP, Hopkins KL, et al. Carbapenem-Resistant Enterobacterales, Carbapenem Resistant Organisms, Carbapenemase-Producing Enterobacterales, and Carbapenemase-Producing Organisms: Terminology Past its "Sell-By Date" in an Era of New Antibiotics and Regional Carbapenemase Epidemiology. Clin Infect Dis 2020;71:1776-82. [Crossref] [PubMed]
- Kois AK, Nicolau DP, Kuti JL. Unresolved issues in the identification and treatment of carbapenem-resistant Gram-negative organisms. Curr Opin Infect Dis 2020;33:482-94. [Crossref] [PubMed]
- Brink AJ. Epidemiology of carbapenem-resistant Gram- negative infections globally. Curr Opin Infect Dis 2019;32:609-16. [Crossref] [PubMed]
- Nordmann P, Poirel L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin Infect Dis 2019;69:S521-8. [Crossref] [PubMed]
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level
- Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin Infect Dis 2019;69:S565-75. [Crossref] [PubMed]
- Lee YL, Chen HM, Hii IM, et al. Carbapenemase-producing Enterobacterales infections: recent advances in diagnosis and treatment. Int J Antimicrob Agents 2022;59:106528. [Crossref] [PubMed]
- Nang SC, Azad MAK, Velkov T, et al. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol Rev 2021;73:679-728. [Crossref] [PubMed]
-
China Antimicrobial Surveillance Network - Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019;39:10-39. [Crossref] [PubMed]
- Falagas ME, Kyriakidou M, Voulgaris GL, et al. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: An evaluation of the current evidence. J Glob Antimicrob Resist 2021;24:342-59. [Crossref] [PubMed]
- Feng JY, Peng CK, Sheu CC, et al. Efficacy of adjunctive nebulized colistin in critically ill patients with nosocomial carbapenem-resistant Gram-negative bacterial pneumonia: a multi-centre observational study. Clin Microbiol Infect 2021;27:1465-73. [Crossref] [PubMed]
- Xia GL, Jiang RL. Efficacy and safety of polymyxin B in carbapenem-resistant gram-negative organisms infections. BMC Infect Dis 2021;21:1034. [Crossref] [PubMed]
- Zeng H, Zeng Z, Kong X, et al. Effectiveness and Nephrotoxicity of Intravenous Polymyxin B in Chinese Patients With MDR and XDR Nosocomial Pneumonia. Front Pharmacol 2020;11:579069. [Crossref] [PubMed]
- Dalyan Cilo B, Topaç T, Ağca H, et al. Comparison of Clinical Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution methods for determining the susceptibilities of Candida isolates. Mikrobiyol Bul 2018;52:35-48. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015;87:62-73. [Crossref] [PubMed]
- Vicari G, Bauer SR, Neuner EA, et al. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin Infect Dis 2013;56:398-404. [Crossref] [PubMed]
- Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram- negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018;18:391-400. [Crossref] [PubMed]
- Tian Y, Zhang Q, Wen L, et al. Combined effect of Polymyxin B and Tigecycline to overcome Heteroresistance in Carbapenem-Resistant Klebsiella pneumoniae. Microbiol Spectr 2021;9:e0015221. [Crossref] [PubMed]
- Li J, Fu Y, Zhang J, et al. Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann Palliat Med 2019;8:651-9. [Crossref] [PubMed]
- Li J, Fu Y, Zhang J, et al. The efficacy of colistin monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii ST2 isolates. J Chemother 2020;32:359-67. [Crossref] [PubMed]
- Falagas ME, Rizos M, Bliziotis IA, et al. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis 2005;5:1. [Crossref] [PubMed]
- Zhang J, Hu Y, Shen X, et al. Risk factors for nephrotoxicity associated with polymyxin B therapy in Chinese patients. Int J Clin Pharm 2021;43:1109-15. [Crossref] [PubMed]
- Zhang X, Qi S, Duan X, et al. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study. J Transl Med 2021;19:431. [Crossref] [PubMed]
- Rigatto MH, Behle TF, Falci DR, et al. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother 2015;70:1552-7. [Crossref] [PubMed]
- Phe K, Lee Y, McDaneld PM, et al. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 2014;58:2740-6. [Crossref] [PubMed]
- Rigatto MH, Vieira FJ, Antochevis LC, et al. Polymyxin B in Combination with Antimicrobials Lacking In Vitro Activity versus Polymyxin B in Monotherapy in Critically Ill Patients with Acinetobacter baumannii or Pseudomonas aeruginosa Infections. Antimicrob Agents Chemother 2015;59:6575-80. [Crossref] [PubMed]
- Cai B, Cai Y, Liew YX, et al. Clinical Efficacy of Polymyxin Monotherapy versus Nonvalidated Polymyxin Combination Therapy versus Validated Polymyxin Combination Therapy in Extensively Drug-Resistant Gram-Negative Bacillus Infections. Antimicrob Agents Chemother 2016;60:4013-22. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


