
Combination of neutrophil-to-lymphocyte ratio and albumin concentration to predict the prognosis of esophageal squamous cell cancer patients undergoing esophagectomy
Highlight box
Key findings
• Pre-operative NLR-Alb is a novel index to predict prognosis of patients with ESCC individually.
What is known and what is new?
• Neutrophil-to-lymphocyte ratio (NLR) has been reported as a prognostic indicator in several tumors including Esophageal cancer (EC). Several meta-analyses have shown that higher NLR is significantly associated with poor outcomes in EC.
• Besides inflammatory factor status, nutritional status can also impact survival of cancer patients. Albumin (Alb) concentration is an easily obtained indicator to reflect nutritional status. Thus, we hypothesized that the combination of NLR and Alb (NLR-Alb) would be an available indicator to predict the prognosis of ESCC and can stratify patients into different risk categories.
What is the implication, and what should change now?
• NLR-Alb is a favorable and cost-effective index to predict prognosis of patients with ESCC.
Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer-related death and the eighth most common cancer worldwide (1). The 5-year survival of EC is approximately 15–25% (2). Esophageal squamous cell cancer (ESCC) is the predominant histological type, especially in China, where the incidence of ESCC is more than 100 cases/100,000 person-years (2,3).
In view of the high incidence and poor prognosis of ESCC, predicting the outcomes in individual patients is crucial. Many studies have suggested that age, gender, tumor differentiation, tumor-node-metastasis (TNM) status, pre-operative therapy, and molecular markers such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and p53 can predict prognosis (4-7). According to a meta-analysis, several tumor biomarkers such as cyclooxygenase-2 (COX-2), p21, p27, cyclin D1, human epidermal growth factor receptor-2 (HER-2), Ki67 can predict prognosis of EC (8). Circulating microRNAs and related microRNA family has been reported as potential prognostic biomarkers (9,10). However, these biomarkers cannot be obtained easily, which limit clinical practice.
Recently, neutrophil-to-lymphocyte ratio (NLR) has been reported as a prognostic indicator in several tumors including EC (11). As an inflammatory and immunological index, NLR can well reflect systemic inflammatory response (SIR), which is associated with tumor progression and metastasis (12). Several meta-analyses have shown that higher NLR is significantly associated with poor outcomes in EC (13,14). Besides inflammatory factor status, nutritional status can also impact survival of cancer patients (15). Albumin (Alb) concentration is an easily obtained indicator to reflect nutritional status. It has been reported that neutrophil lymphocyte ratio/albumin ratio (NLR/Alb) was a prognostic index for ESCC (16,17). However, these studies did not stratify patients into different risk categories. A retrospective study reported Alb concentration combined with NLR (COA-NLR) can predict overall survival of patients with non-small cell lung cancer and stratify patients into 3 risk categories (18). Thus, in this paper we divided patients into 3 cohort via the combination of NLR and Alb (NLR-Alb), and we hypothesized that pre-operative NLR-Alb would be an available indicator to predict the prognosis of ESCC patients who underwent esophagectomy more accurately. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-333/rc).
Methods
Participants
A total of 366 patients with ESCC who accepted esophagectomy at Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, China, from December 2007 to December 2010 were recruited to this study. All cases were diagnosed by pathology and classified via the 8th edition of the TNM classification (19). Patients with autoimmune disease, hematological disease, infection, and immunotherapy history were excluded (Figure 1). Some patients had received chemotherapy and/or radiotherapy before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants provided written informed consent. The study was approved by the Ethics Committee of Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital (No. K2023-025-01).
Study design
This was a retrospective study. We collected the demographic data, pathologic findings, TNM stage, surgical type, blood cell count, and serum Alb concentration of 366 cases. The laboratory data within 1 week before surgery between 6 am and 10 am were collected. The survival data were collected via medical records or telephone, with 31 December 2016 being the deadline of the follow-up. Overall survival (OS) was defined as the time from the surgery to death or last follow-up. We investigated the relationship of NLR-Alb with survival via univariate and multivariate analysis. Moreover, we compared clinical features among NLR-Alb cohorts.
Definition
NLR was defined as absolute neutrophil count/absolute lymphocyte count. The NLR and Alb threshold were calculated based on the predominant point on receiver operating characteristic (ROC) curves. the 2 parameters threshold values were 1.9 [sensitivity: 0.678, specificity: 0.786, the area under ROC curve (AUC): 0.632] and 39 g/L (sensitivity: 0.977, specificity: 0.025, AUC: 0.652), respectively. The calculation of NLR-Alb is shown in Figure 2. Patients with a lower NLR (≤1.9) and a higher Alb (>39 g/L) were allocated to the NLR-Alb 1 cohort. Patients with a higher NLR (>1.9) and a lower Alb (≤39 g/L) were allocated to the NLR-Alb 3 cohort. The remaining patients were allocated to the NLR-Alb 2 cohort.
Statistical analysis
The one-way analysis of variance (ANOVA) test was used to compare co-variates among the cohorts. Comparison of enumeration data used the chi-squared (χ2) test. The cutoff values were set as the point on the ROC curves that was closest to the upper left-hand corner of the plot. Kaplan-Meier curve was used to analyze OS, and comparisons were determined by log-rank test. Each variable was assessed on the univariate analysis, and then calculated on the multivariable Cox proportional hazards model to validate their independent prognostic value. A P value <0.05 was considered statistically significant. Data were analyzed as of 2022 using the software SPSS 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The baseline characteristics of 366 subjects are shown in Table 1. There were 285 (78%) males and 81 (22%) females with a median age of 56 (range, 35–86) years. The median body mass index (BMI) was 22.3 (range, 14.7–37.3) kg/m2. More than half of the patients (53%) did not accept pre-operative therapy. A total of 194 (53%) patients were in TNM stage III–IV. There were no significant differences in age, gender, BMI, smoking, alcohol, pre-operative therapy, surgical type, tumor location, or tumor status among the NLR-Alb cohorts.
Table 1
| Variables | Total =366 | NLR-Alb 1 (n=70) | NLR-Alb 2 (n=220) | NLR-Alb 3 (n=76) | P value |
|---|---|---|---|---|---|
| Age, year (median, range) | 56 [35–86] | 54 [37–76] | 57 [35–86] | 57 [39–81] | 0.06 |
| Gender | 0.97 | ||||
| Male | 285 (78%) | 54 (77%) | 171 (78%) | 60 (79%) | |
| Female | 81 (22%) | 16 (23%) | 49 (22%) | 16 (21%) | |
| BMI, kg/m2 (median, range) | 22.3 (14.7–37.3) | 22.3 (15.5–31.6) | 22.2 (14.7–33.1) | 23.2 (16.3–37.3) | 0.23 |
| Smoking | 140 (38%) | 27 (39%) | 87 (40%) | 26 (34%) | 0.73 |
| Alcohol | 51 (14%) | 8 (11%) | 35 (16%) | 8 (11%) | 0.47 |
| Preoperative therapy | 0.38 | ||||
| Chemotherapy | 124 (34%) | 24 (34%) | 76 (35%) | 24 (32%) | |
| Radiotherapy | 17 (5%) | 1 (1%) | 9 (4%) | 7 (9%) | |
| Chemo-radiotherapy | 27 (7%) | 4 (6%) | 20 (9%) | 3 (4%) | |
| Else | 3 (1%) | 0 | 2 (1%) | 1 (1%) | |
| None | 195 (53%) | 41 (59%) | 113 (51%) | 41 (54%) | |
| Surgical type | 0.20 | ||||
| Three-field lymphadenectomy | 286 (78%) | 53 (76%) | 171 (78%) | 62 (82%) | |
| Two-field lymphadenectomy | 68 (19%) | 16 (23%) | 38 (17%) | 14 (18%) | |
| Left thorax | 12 (3%) | 1 (1%) | 11 (5%) | 0 | |
| Tumor location | 0.35 | ||||
| Upper third | 65 (18%) | 13 (19%) | 34 (16%) | 18 (24%) | |
| Middle third | 264 (72%) | 53 (76%) | 161 (73%) | 50 (66%) | |
| Lower third | 37 (10%) | 4 (6%) | 25 (11%) | 8 (11%) | |
| Tumor differentiation | 0.48 | ||||
| Well | 41 (11%) | 5 (7%) | 25 (11%) | 11 (14%) | |
| Moderate | 285 (78%) | 56 (80%) | 169 (77%) | 60 (79%) | |
| Poor | 40 (11%) | 9 (13%) | 26 (12%) | 5 (7%) | |
| Pathologic N stage | 0.38 | ||||
| N0 | 187 (51%) | 42 (60%) | 111 (51%) | 34 (45%) | |
| N1 | 126 (34%) | 22 (31%) | 73 (33%) | 31 (41%) | |
| N2 | 48 (13%) | 6 (9%) | 33 (15%) | 9 (12%) | |
| N3 | 5 (2%) | 0 | 3 (1%) | 2 (3%) | |
| Pathologic T stage | 0.92 | ||||
| T1 | 59 (16%) | 13 (19%) | 34 (16%) | 12 (16%) | |
| T2 | 64 (18%) | 10 (14%) | 39 (18%) | 15 (20%) | |
| T3 | 176 (48%) | 36 (51%) | 103 (47%) | 37 (49%) | |
| T4 | 67 (18%) | 11 (16%) | 44 (20%) | 12 (16%) | |
| Metastasis | 1.00 | ||||
| M0 | 365 (99%) | 70 (100%) | 219 (99%) | 76 (100%) | |
| M1 | 1 (1%) | 0 | 1 (1%) | 0 | |
| TNM status | 0.69 | ||||
| I–II | 172 (47%) | 36 (51%) | 102 (46%) | 34 (45%) | |
| III–IV | 194 (53%) | 34 (49%) | 118 (54%) | 42 (55%) | |
| OS, months (median, range) | 76.4 (0.4–102.4) | 82.8 (10.6–101.4) | 76.3 (0.4–102.4) | 62.5 (1.71–98.5) | <0.001 |
NLR-Alb, combination of neutrophil-to-lymphocyte ratio and albumin concentration; BMI, body mass index; TNM, tumor-node-metastasis; OS, overall survival.
Prognostic analysis
Univariate analysis showed that age (P=0.013), gender (P=0.021), surgical type (P=0.031), preoperative therapy (P=0.007), NLR-Alb (P=0.001), and TNM status (P<0.001) were associated with 5-year OS. In multivariate analysis, NLR-Alb [hazard ratio (HR) =2.53, 95% confidence interval (95% CI): 1.38–4.63, P=0.003] and TNM status (HR =4.76, 95% CI: 3.09–7.33, P<0.001) were independent predictive factors for 5-year OS (Table 2).
Table 2
| Variables | Univariate analysis for OS | Multivariate analysis for OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | |||||||
| <65 | Ref | Ref | Ref | Ref | |||
| ≥65 | 1.622 | 1.108–2.374 | 0.013 | 1.392 | 0.928–2.087 | 0.110 | |
| Gender | |||||||
| Male | Ref | Ref | Ref | Ref | |||
| Female | 0.572 | 0.355–0.920 | 0.021 | 0.719 | 0.445–1.163 | 0.179 | |
| BMI, kg/m2 | |||||||
| <19.6 | Ref | Ref | |||||
| ≥19.6 | 0.790 | 0.527–1.182 | 0.251 | ||||
| Surgical type | 0.031 | 0.397 | |||||
| Three-field lymphadenectomy | Ref | Ref | Ref | Ref | |||
| Two-field lymphadenectomy | 0.549 | 0.325–0.929 | 0.025 | 0.748 | 0.439–1.274 | 0.285 | |
| Left thorax | 1.667 | 0.733–3.793 | 0.223 | 1.407 | 0.596–3.324 | 0.436 | |
| Tumor location | 0.246 | ||||||
| Upper third | Ref | Ref | |||||
| Middle third | 0.834 | 0.535–1.300 | 0.423 | ||||
| Lower third | 1.273 | 0.688–2.358 | 0.442 | ||||
| Preoperative therapy | 1.784 | 1.259–2.530 | 0.007 | 1.335 | 0.912–1.954 | 0.137 | |
| Smoking | 1.100 | 0.775–1.561 | 0.594 | ||||
| Alcohol | 1.123 | 0.690–1.827 | 0.641 | ||||
| NLR-Alb | 0.001 | <0.001 | |||||
| NLR-Alb 1 | Ref | Ref | Ref | Ref | |||
| NLR-Alb 2 | 2.598 | 1.419–4.758 | 0.002 | 2.530 | 1.381–4.634 | 0.003 | |
| NLR-Alb 3 | 3.469 | 1.795–6.701 | <0.001 | 3.795 | 1.962–7.339 | <0.001 | |
| Alb, g/L | |||||||
| <39 | Ref | Ref | |||||
| ≥39 | 0.866 | 0.590–1.272 | 0.866 | ||||
| TNM | |||||||
| I–II | Ref | Ref | Ref | Ref | |||
| III–IV | 4.645 | 3.019–7.146 | <0.001 | 4.761 | 3.091–7.334 | <0.001 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; NLR-Alb, combination of neutrophil-to-lymphocyte ratio and albumin concentration; TNM, tumor-node-metastasis.
Outcomes
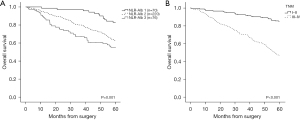
The median OS of 366 cases was 76.4 months (range, 0.4–102.4 months), among them the NLR-Alb 1 cohort had the longest median OS, which was 82.8 months (range, 10.6–101.4 months) compared to 76.3 months (range, 0.4–102.4 months) in the NLR-Alb 2 cohort and 62.5 months (range, 1.71–98.5 months) in the NLR-Alb 3 cohort (P<0.001, Table 1). The 5-year OS rates were 83%, 62%, and 55% for NLR-Alb 1, NLR-Alb 2, and NLR-Alb 3, respectively (P=0.001, Figure 3A). The 5-year OS rates between TNM I–II and III–IV were 85% and 46% (P<0.001, Figure 3B).
Discussion
ESCC is an aggressive cancer with a high incidence and mortality. It is crucial to predict prognosis of these patients individually so they can receive appropriate treatment and better outcomes. The emerging role of pre-operative inflammatory and nutritional status in prognosis is a popular topic in several solid tumors. Except for TNM status, NLR, platelet-to-lymphocyte ratio (PLR), C-reactive protein (CRP), serum Alb, and BMI have been seen to be significant prognostic factors in survival of ESCC (20-23).
SIR plays an important role in the development and progression of tumors through tumor associated inflammation, chronic inflammation, and infection autoimmunity, moreover, SIR can suppress antitumor immunity and stimulate angiogenesis causing metastasis (12). CRP, procalcitonin (PCT), and cytokines can reflect status of SIR. However, they can be affected by many factors with low specificity. The NLR, as an easily calculated and cost-effective objective index, can reveal the status of SIR and be a marker of immunosurveillance failure. Many cytokines produced by neutrophils such as interleukin-6 (IL-6), IL-1, tumor necrosis factor (TNF) and VEGF may enhance tumor growth (24). The increased number of neutrophils can inhibit the antitumor activity of natural killer (NK) and activated T cells (25,26). In addition, cancer immunosurveillance is mediated by CD-4 helper lymphocytes and CD-8 suppressor lymphocytes. The decrease of CD-4 T cells and increase of CD-8 T cells lead to the depression of cancer cell destruction (27). The decreased number of lymphocytes result in immunosuppression causing production of inflammatory cytokines in the tumor microenvironment (28). A study which enrolled 68 ESCC patients who underwent definitive concurrent chemoradiotherapy showed that higher post-therapy NLR was associated with poorer OS (median 9.4 vs.15.2 months, P=0.03) (29). Studies have shown that among patients with EC who underwent surgery, lower pre-treatment NLR was also associated with disease-free survival (DFS) or OS (30-32). Moreover, a study reported that preoperative NLR was an independent variable associated with the development of postoperative complications (33).
A low Alb concentration is associated with poor prognosis because hypoalbuminemia indicates a poor nutritional status and existence of SIR (34-36). However, the serum Alb concentration was not an independent prognostic factor in our study (HR =0.87, 95% CI: 0.59–1.27, P=0.866).
For predicting prognosis accurately and individually, we used the combination of NLR and Alb to investigate the outcome in patients with ESCC. NLR and serum Alb concentration can be obtained easily during routine blood test, and are cost-effective. NLR-Alb can reveal not only SIR status but also nutritional status. We classified cases into 3 cohorts via NLR-Alb score, and found that a low NLR-Alb score was relevant to a better outcome. Moreover, NLR-Alb was shown to be an independent prognostic indicator for patients with ESCC via multivariate analyses. As shown in Table 1, the 3 cohorts divided according to NLR-Alb had no significant differences in TNM status, therefore, NLR-Alb was an independent factor with little effect by TNM status to predict prognosis. In our study, univariate analysis showed that age, gender, surgical type, preoperative therapy, NLR-Alb, and TNM status were associated with 5-year OS. Multivariate analysis showed that the higher NLR-Alb score and TNM III–IV status were associated with poor outcomes. The older patients usually had worse body condition with poor pulmonary function, cardiac function, or renal function before surgery, which may impact prognosis. The patients who accepted pre-operative therapy usually had poor TNM stages, and it was necessary to shrink the tumor by pro-operative therapy before esophagectomy. In addition, the side effect of pre-operative therapy may impact patients’ organ function causing unfavorable outcomes.
Our study had important limitations. It was a single center, retrospective study. The bias of patient number among the 3 cohorts was unsatisfactory, with more cases in the NLR-Alb 2 cohort (n=220) compared to other 2 cohorts (n=70, n=76).
Conclusions
In summary, pre-operative NLR-Alb is a favorable and cost-effective index to predict prognosis of patients with ESCC individually, which can stratify patients into different risk categories. In addition, the calculation and obtaining of NLR-Alb is simple, so that NLR-Alb can be easily applied in daily practice.
Acknowledgments
The authors appreciate the academic support from the AME Esophageal Squamous Cell Cancer Collaborative Group.
Funding: This study was supported by the Fujian Provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (No. 2020Y2012) and Research Projects of Fujian Cancer Hospital (No. 2021YN09).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-333/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-333/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-333/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-333/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants provided written informed consent. The study was approved by the Ethics Committee of Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital (No. K2023-025-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21:7933-43. [Crossref] [PubMed]
- Qiu MJ, Yang SL, Wang MM, et al. Prognostic evaluation of esophageal cancer patients with stages I-III. Aging (Albany NY) 2020;12:14736-53. [Crossref] [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Chen M, Cai E, Huang J, et al. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:1126-34. [Crossref] [PubMed]
- Wang XL, Zhang CM, Shi LY, et al. Significance of p53 gene mutation and P53 protein expression abnormality on the prognosis of esophageal cancer: a meta-analysis study. Zhonghua Liu Xing Bing Xue Za Zhi 2004;25:769-74. [PubMed]
- Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer 2013;13:539. [Crossref] [PubMed]
- Liu F, Tian T, Xia LL, et al. Circulating miRNAs as novel potential biomarkers for esophageal squamous cell carcinoma diagnosis: a meta-analysis update. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Yuan L, Bing Z, Yan P, et al. Integrative data mining and meta-analysis to investigate the prognostic role of microRNA-200 family in various human malignant neoplasms: A consideration on heterogeneity. Gene 2019;716:144025. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Ishibashi Y, Tsujimoto H, Yaguchi Y, et al. Prognostic significance of systemic inflammatory markers in esophageal cancer: Systematic review and meta-analysis. Ann Gastroenterol Surg 2020;4:56-63. [Crossref] [PubMed]
- Pirozzolo G, Gisbertz SS, Castoro C, et al. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis 2019;11:3136-45. [Crossref] [PubMed]
- Mantzorou M, Koutelidakis A, Theocharis S, et al. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr Cancer 2017;69:1151-76. [Crossref] [PubMed]
- Zhao Q, Chen S, Feng JF. A novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: neutrophil lymphocyte ratio/albumin ratio. Oncotarget 2017;8:103535-42. [Crossref] [PubMed]
- Lv Y, Zhang J, Liu Z, et al. A novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: Neutrophil lymphocyte ratio/prealbumin ratio. Medicine (Baltimore) 2019;98:e14562. [Crossref] [PubMed]
- Weng J, Huang J, Yu W, et al. Combination of albumin concentration and neutrophil-to-lymphocyte ratio for predicting overall survival of patients with non-small cell lung cancer. J Thorac Dis 2021;13:5508-16. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Miyazaki T, Sakai M, Sohda M, et al. Prognostic Significance of Inflammatory and Nutritional Parameters in Patients with Esophageal Cancer. Anticancer Res 2016;36:6557-62. [Crossref] [PubMed]
- Chen MF, Hsieh CC, Chen PT, et al. Role of Nutritional Status in the Treatment Outcome for Esophageal Squamous Cell Carcinoma. Nutrients 2021;13:2997. [Crossref] [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. [Crossref] [PubMed]
- Xu GW, Wu HR, Xiong R, et al. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thorac Cancer 2018;9:1707-15. [Crossref] [PubMed]
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. [Crossref] [PubMed]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011;71:2411-6. [Crossref] [PubMed]
- Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol 1988;141:4395-402. [Crossref] [PubMed]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006;6:836-48. [Crossref] [PubMed]
- Kwilas AR, Donahue RN, Tsang KY, et al. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron 2015;2:e677. [PubMed]
- Hoshino S, Takeuchi M, Kawakubo H, et al. Usefulness of Neutrophil to Lymphocyte Ratio at Recurrence for Predicting Long-Term Outcomes in Patients with Recurrent Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2021;28:3001-8. [Crossref] [PubMed]
- Sakin A, Alay M, Sahin S, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. North Clin Istanb 2021;8:435-42. [PubMed]
- Kato T, Oshikiri T, Goto H, et al. Preoperative neutrophil-to-lymphocyte ratio predicts the prognosis of esophageal squamous cell cancer patients undergoing minimally invasive esophagectomy after neoadjuvant chemotherapy. J Surg Oncol 2021;124:1022-30. [Crossref] [PubMed]
- Dal F, Topal U, Sozuer E, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with curative resection of esophageal cancer. A single center experience. Ann Ital Chir 2021;92:242-8. [PubMed]
- Shi BW, Xu L, Gong CX, et al. Preoperative Neutrophil to Lymphocyte Ratio Predicts Complications After Esophageal Resection That can be Used as Inclusion Criteria for Enhanced Recovery After Surgery. Front Surg 2022;9:897716. [Crossref] [PubMed]
- Xu XL, Yu HQ, Hu W, et al. A Novel Inflammation-Based Prognostic Score, the C-Reactive Protein/Albumin Ratio Predicts the Prognosis of Patients with Operable Esophageal Squamous Cell Carcinoma. PLoS One 2015;10:e0138657. [Crossref] [PubMed]
- Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol 2015;41:1324-32. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol 2015;22:302-10. [Crossref] [PubMed]
(English Language Editor: J. Jones)




