
An update on red blood cell transfusion in non-cardiac thoracic surgery
Highlight box
Key findings
• The overall rate of red blood cell transfusion in non-cardiac thoracic surgery is low (7.4%).
• Particularly low transfusion rates are observed in patients with lung resections (2.4%).
• Transfusion rates remain high in empyema cases (44.7%) and open surgery (20.0%).
What is known and what is new?
• Previous studies have reported high overall transfusion rates for thoracic procedures.
• In this study, independent risk factors for red blood cell transfusion were empyema (P=0.001), open surgery (P<0.001), low preoperative hemoglobin (P=0.001) and patient age (P=0.013).
What is the implication, and what should change now?
• Preoperative ordering of blood products should be tailored to patient specific risk factors.
Introduction
The supply and transfusion of blood products is a key factor in any major surgery. This involves considerable logistics, surveillance, and costs. It also carries ethical implications, as blood units are a limited resource in all health care systems. During the recent COVID-19 pandemic, blood donations were compromised, endangering the blood supply (1).
While the transfusion of red blood cell (RBC) concentrates is life-saving in certain clinical conditions, it is also associated with short- and long-term adverse events, including worse oncological outcomes in patients with lung cancer (2-5). Hence, there is consensus that it is of utmost importance to critically review the practice of RBC transfusion on a regular basis.
High-quality data on the use of blood products in thoracic surgery often come from patients undergoing cardiac procedures (6,7). However, data on RBC transfusion in non-cardiac thoracic surgery are traditionally scarce and often outdated. Previous non-cardiac studies reported high transfusion rates, e.g., 16.1% for general thoracic procedures, 20.5% for wedge resection, 23.6% for anatomic lung resection, 13.0–19.9% for lobectomy, and up to 25.0% for pneumonectomy (8-11). These numbers do not match current clinical observations.
Therefore, this study sought to evaluate recent data on the transfusion practice of RBC concentrates in a cohort of non-cardiac thoracic surgery patients at one tertiary referral center. According to the Society of Thoracic Surgeons’ (STS) current guideline on patient blood management, early identification of high-risk patients is vital, as those account for the majority of blood transfusions. For this reason, we tried to detect patient-specific risk factors for the transfusion of RBC concentrates (12). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1581/rc).
Methods
Ethical review board approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics review board approval (No. 2021-15979, date: 17.07.2021) was obtained from the ethics commission of the State Chamber of Physicians in Rhineland-Palatinate in Mainz, Germany. The review board waived the need for patient consent for this retrospective study.
Patients
All consecutive patients operated in the Department of Thoracic Surgery, University Medical Center Mainz, Germany, between January and December 2021 were identified for this monocentric retrospective study in a tertiary referral center. Patients undergoing elective and urgent surgery, as well as patients requiring intensive care for their postoperative treatment were included. Patients that were referred for thoracic surgery from other departments and had been previously treated there as inpatients were excluded. Patients undergoing minor procedures (chest tube insertion under local anesthesia, minor wound care) or patients with extra-thoracic procedures were excluded.
Patient blood management
Institutional patient blood management protocols were applicable to all patients in this study, which included perioperative measures for managing anemia, optimizing coagulation and using blood conservation strategies. Regarding drug anticoagulation, acetylsalicylic acid (ASC) was continued perioperatively in all elective cases. In cases with double platelet inhibition (ASC plus clopidogrel), clopidogrel was paused in a timely manner prior to surgery when feasible. In patients with therapeutic anticoagulation, the drug was paused prior to surgery according to the respective agent, i.e., 24–48 hours for modern oral anticoagulants (apixaban, rivaroxaban, edoxaban) or according to international normalized ratio (INR) values for coumarin derivatives (where bridging with heparin was performed). Hemoglobin levels, thrombocyte levels and INR were monitored perioperatively in all patients and corrected following institutional protocols and the German cross-sectional guidelines for therapy with blood components and plasma derivatives, amended ed., 2020 (13).
Blood ordering schedule and indications for RBC transfusion
For all patients scheduled for major thoracic surgery, two units of RBC were routinely cross-matched prior to surgery. More units were requested only in select high-risk cases (e.g., resections on cardiopulmonary bypass or vascular reconstruction). Type and antibody screen only were not routinely performed to avoid a loss of time in urgent cases. Indications for RBC transfusion were severe perioperative bleeding and the prevention of tissue hypoxemia according to national and institutional guidelines for hemotherapy (13). In accordance with the guideline, a restrictive transfusion strategy was pursued, which has been shown to be associated with improved outcomes in surgical patients (14).
Data acquisition
Data on blood ordering and RBC transfusion had been previously collected for internal quality control. Transfusion data were cross-checked using a retrospective review of the electronic documentation system of medical procedures at our institution. The presented numbers of cross-matched RBC units are the numbers requested for cross-matching prior to surgery. Results of type and antibody screen were extracted from the electronic laboratory system of our institution’s blood bank. Patients were classified as “transfused” if they received at least one unit of RBC between the start of surgery and postoperative day 3. This time interval was chosen to detect transfusion events likely related to the procedure. Anticoagulation was defined as the administration of any anticoagulant that had not been discontinued before surgery as recommended by our institutional guidelines. Venous thromboembolism medication in prophylactic dosage alone (e.g., low-molecular-weight heparin) was not considered anticoagulation. The thoracic morbidity and mortality classification system was used to grade surgical complications (15). Major morbidity was defined as complications ≥ grade 3. Mortality was recorded as in-hospital mortality. Tumor stages were assessed using the Union for International Cancer Control staging system for non-small cell lung cancer (8th ed., 2017).
Statistical analysis
The median is presented with the interquartile range for quantitative variables; qualitative variables are shown as absolute numbers and relative frequencies. For bivariate analysis of categorical variables χ2 or Fisher’s exact test was used, as appropriate. Bivariate analysis of continuous variables was carried out with independent samples Student’s t-test. The Cochran-Armitage test for trend was used to identify the association between a binary variable and an ordinal variable with >2 categories. A test result was considered statistically significant if P<0.05. For the binary outcome “RBC transfusion”, a multiple logistic regression analysis was conducted. Variables were entered into the model if P<0.10 in a univariable analysis. In the multiple analysis, the backward stepwise selection based on the probability of the Wald statistic was used and a significance level of α=0.05 was chosen to determine final independent predictors. Odds ratios are presented together with their 95.0% confidence intervals (CIs). A receiver operating characteristic (ROC) analysis was used to determine cut-off values for the variables “preoperative hemoglobin”, “age”, and “length of surgery” to separate patients with versus those without RBC transfusion. All statistical tests were two-tailed. The complete case approach was used for missing data. Analyses were performed using SPSS Statistics software (version 26, IBM, Armonk, NY, USA).
Results
A brief summary of the main findings of the study is shown in Figure 1.
Patients and surgical procedures
Between January and December 2021, a total of 379 patients met the inclusion criteria. A flowchart of the study population is shown in Figure 2. Most patients were male (54.4%) and were categorized as American Society of Anesthesiologists physical status class 3 (55.2%), with a median age of 65 years. Therapy with anticoagulants at the time of surgery was present in 19.5% of cases. Table 1 shows the patients’ clinical characteristics and surgical outcomes. Most cases were elective (72.6%) and were operated using a minimally invasive [i.e., video-assisted thoracoscopic surgery (VATS) or robot-assisted thoracic surgery (RATS)] approach (72.4%). Half (50.7%) of all patients were operated for cancer. All patients who underwent surgery for empyema underwent thoracoscopic or open decortication. The overall median length of surgery was 72 minutes, major morbidity and mortality occurred in 5.5% and 1.6%, respectively. Surgical revision was necessary in 2.5% of cases. None of the revisions were due to hemorrhage.
Table 1
| Variables | Values | Missing data (n) |
|---|---|---|
| Female | 173 (45.6) | 0 |
| Age (years) | 65 [53–74] | 0 |
| BMI (kg/m2) | 25.4 [22.0–29.6] | 22 |
| Malignant disease | 192 (50.7) | 0 |
| Coagulopathy | 11 (2.9) | 0 |
| Anticoagulation | 74 (19.5) | 4 |
| ASA | 31 | |
| 1 | 3 (0.9) | – |
| 2 | 101 (29.0) | – |
| 3 | 192 (55.2) | – |
| 4 | 52 (14.9) | – |
| Preoperative laboratory data | ||
| Hb (13.5–17.5 g/dL) | 12.8 [11.1–14.3] | 1 |
| Thrombocytes (150–360/nL) | 271 [199–359] | 2 |
| Leucocytes (×109/L) | 7.7 [6.2–9.8] | 2 |
| CRP (mg/L) | 10.0 [2.5–49.5] | 6 |
| Creatinine (mg/dL) | 0.8 [0.7–1.0] | 2 |
| APTT (25–37 sec) | 30.0 [27.8–32.1] | 3 |
| INR | 1.1 [1.0–1.2] | 2 |
| Outcomes of surgery | ||
| Length of surgery (min) | 72 [44–144] | 0 |
| Major morbidity (TM&M ≥3) | 15 (5.5) | 0 |
| Mortality | 6 (1.6) | 0 |
| UICC stage (NSCLC) | 0 | |
| IA1 | 7 (10.0) | – |
| IA2 | 20 (28.6) | – |
| IA3 | 9 (12.9) | – |
| IB | 2 (2.9) | – |
| IIA | 1 (1.4) | – |
| IIB | 5 (7.1) | – |
| IIIA | 9 (12.9) | – |
| IIIB | 3 (4.3) | – |
| IVA | 9 (12.9) | – |
| IVB | 5 (7.1) | – |
| R classification (NSCLC) | 0 | |
| R0 | 64 (91.4) | – |
| R+ | 6 (8.6) | – |
Data are shown as n (%) or median [IQR]. BMI, body mass index; ASA, American Society of Anesthesiologists; Hb, hemoglobin; CRP, C-reactive protein; APTT, activated partial thromboplastin time; INR, international normalized ratio; TM&M, thoracic morbidity & mortality classification; UICC, Union for international cancer control; NSCLC, non-small cell lung cancer; IQR, interquartile range.
Perioperative transfusion of RBC concentrates
Data on cross-matching and RBC transfusion are shown in Table 2. Preoperative crossmatch was requested in 328 (86.5%) patients. A total of 28 (7.4%) patients received an RBC transfusion. Overall, 916 RBC units were cross-matched preoperatively, and 68 RBC units were transfused between the beginning of surgery and postoperative day 3 (cross match to transfusion ratio: 13.5). A median number of two units were given per patient. Figure 3A shows the distribution of units transfused in the study cohort; and the timing of transfusion is shown in Figure 3B. Only one case was identified in which no cross-matched units had been ordered preoperatively yet the patient required perioperative RBC transfusion. This patient underwent a minor thoracoscopic procedure when intraoperative bleeding from the internal thoracic artery occurred, requiring conversion to open surgery to control bleeding. Intraoperative transfusion of four RBC units was required. No adverse events related to the transfusion of RBC concentrates were observed in any of the 28 patients in the transfusion group.
Table 2
| Variables | Values | Missing data (n) |
|---|---|---|
| Patients cross-matched | 328 (86.5) | 0 |
| Units/patient cross-matched | 2 [2–4] | 0 |
| Patients transfused | 28 (7.4) | 0 |
| Units/patient transfused | 2 [1–4] | 0 |
| Transfusion probability† | 8.5 | – |
| Crossmatch/transfusion ratio‡ | 13.5 | – |
| Transfusion index§ | 0.2 | – |
| Antigens | ||
| Rh | 271 (82.9) | 0 |
| Cw | 11 (3.4) | 0 |
| Kell | 25 (2.6) | 0 |
| Antibodies | 25 (7.6) | 0 |
Data are shown as n (%) or median [IQR]. †, number of patients transfused/number of patients cross-matched ×100; ‡, number of units cross-matched/number of units transfused; §, number of units transfused/number of patients cross-matched. RBC, red blood cell; IQR, interquartile range.
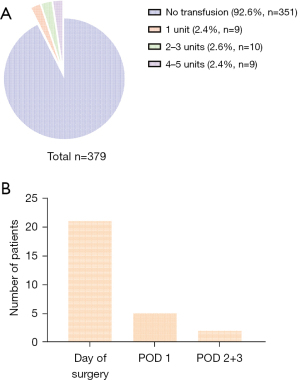
Surgical procedures and transfusion rates
Table 3 shows RBC transfusion rates according to surgical procedures and other distinct parameters. Higher transfusion rates were observed in non-elective (20.2%) when compared to elective (2.5%) procedures. Similarly, higher transfusion rates were observed in patients who underwent open surgery (20.0%) when compared to patients operated with a thoracoscopic or robotic-assisted approach (2.7%). Patients undergoing lung resections had an overall transfusion rate of 2.4% (wedge resection: 1.4%, lobectomy: 5.2%), whereas patients undergoing other procedures received RBC transfusion in 11.4% of cases. Patients undergoing empyema surgery had the highest observed transfusion rate (44.7%) and, in turn, a nearly 10-fold increased risk for transfusion when compared to lobectomy. In-hospital mortality was not different between patients with and without RBC transfusion (P=0.36).
Table 3
| Variables | Values | RBC transfusion rate (%) |
|---|---|---|
| Calculated cut-off values | ||
| Hb (<10.4 g/dL)† | 71 (18.8) | 32.4 |
| Age (>77 years)† | 58 (15.3) | 17.2 |
| Length of surgery (>108 min)† | 134 (35.4) | 12.7 |
| Type of surgery | ||
| Open surgery‡ | 100 (27.6) | 20.0 |
| VATS/RATS | 262 (72.4) | 2.7 |
| Non-elective | 104 (27.4) | 20.2 |
| Elective | 275 (72.6) | 2.5 |
| Redo procedure | 18 (4.7) | 11.1 |
| Surgical procedures | ||
| Lung resections | 169 (44.6) | 2.4 |
| Lobectomy | 58 (15.3) | 5.2 |
| Segmentectomy | 14 (3.7) | 0.0 |
| Pneumonectomy | 7 (1.8) | 0.0 |
| Wedge resection | 70 (18.5) | 1.4 |
| Metastasectomy§ | 20 (5.3) | 0.0 |
| Other procedures | 210 (55.5) | 11.4 |
| Empyema (decortication) | 38 (10.0) | 44.7 |
| Mediastinum | 23 (6.1) | 13.0 |
| Chest wall | 28 (7.4) | 7.1 |
| Hemothorax | 17 (4.5) | 5.9 |
| Pleura | 62 (16.4) | 1.6 |
| Pneumothorax | 25 (6.6) | 0.0 |
| Other | 17 (4.5) | 0.0 |
Data are shown as n (%). †, cut-off values determined by ROC analysis; ‡, including conversion; §, precision excision (electrocautery or laser assisted). Hb, hemoglobin; VATS, video-assisted thoracic surgery; RATS, robot-assisted thoracic surgery; RBC, red blood cell; ROC, receiver operating characteristic.
Risk factors for RBC transfusion
Results of univariable and multivariable analysis are shown in Table 4. In univariable analysis, surgery for empyema (P<0.001), open surgery (P<0.001), lower preoperative hemoglobin levels (P<0.001), older patient age (P=0.02), higher American Society of Anesthesiologists class (P<0.001), non-elective surgery (P<0.001), longer operating time (P=0.006), therapy with anticoagulants at the time of surgery (P=0.04), higher preoperative levels of thrombocytes (P=0.04), leucocytes (P=0.04), and C-reactive protein (P<0.001), as well as higher INR values (P=0.001) were significantly associated with RBC transfusion. In multivariable analysis, surgery for empyema (P=0.001), lower preoperative hemoglobin levels (P=0.001), older patient age (P=0.013), and open surgery (P<0.001) were the only independent risk factors for RBC transfusion.
Table 4
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| P value | P value | Odds ratio (95.0% CI) | ||
| Empyema | <0.001* | 0.001* | 15.708 (3.106–79.450) | |
| Open surgery | <0.001* | <0.001* | 26.413 (5.520–126.373) | |
| Hb | <0.001* | 0.001* | 0.524 (0.358–0.768) | |
| Age | 0.02* | 0.013* | 1.068 (1.014–1.125) | |
| ASA | <0.001* | 0.2 | – | |
| Non-elective surgery | <0.001* | 0.97 | – | |
| Length of surgery | 0.006* | 0.07 | – | |
| Anticoagulation | 0.04* | 0.59 | – | |
| Thrombocytes | 0.04* | 0.88 | – | |
| Leukocytes | 0.04* | 0.10 | – | |
| CRP | <0.001* | 0.81 | – | |
| INR | 0.001* | 0.31 | – | |
| APTT | 0.39 | – | – | |
| Creatinine | 0.78 | – | – | |
| Gender | 0.32 | – | – | |
| BMI | 0.53 | – | – | |
| Coagulopathy | 0.57 | – | – | |
| Redo procedure | 0.63 | – | – | |
| UICC stage (NSCLC) | 0.92 | – | – | |
| R classification (NSCLC) | >0.99 | – | – | |
| Blood type | 0.44 | – | – | |
Asterisks (*) indicate statistical significance. All variables with P<0.10 in the univariable analyses were entered into the multivariable analysis. In multivariable analysis, only empyema, open surgery, Hb and age remained in the model as independent prognostic factors. RBC, red blood cell; APTT, activated partial thromboplastin time; ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; Hb, hemoglobin; INR, international normalized ratio; UICC, Union for International Cancer Control; NSCLC, non-small cell lung cancer.
Cut-off values for independent risk factors
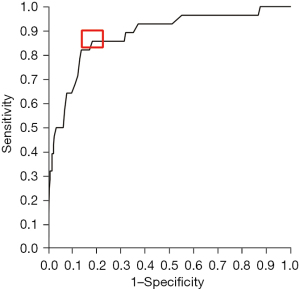
To determine optimal cut-off values for the independent predictors “preoperative hemoglobin” and “patient age” in multivariable analysis, ROC analyses were performed. For preoperative hemoglobin, the highest Youden’s J was observed for values <10.4 g/dL with a sensitivity of 82.1% and a specificity of 86.3%. The area under the curve was 0.882 (95.0% CI: 0.810–0.955). The corresponding ROC curve is shown in Figure 4. The optimal cut-off value for patient age was >76.5 years (sensitivity 39.3%, specificity 83.5%) with an area under the curve of 0.627 (95.0% CI: 0.520–0.733). The parameter “length of surgery“ was not an independent predictor in multivariable analysis; however, the best value to separate patients with versus those without RBC transfusion was >108 min (sensitivity 64.3%, specificity 66.4%, AUC 0.655 with a 95.0% CI of 0.557–0.753).
Discussion
Our study shows current transfusion data in the era of minimally invasive surgery in a cohort comprising the typical spectrum of modern thoracic procedures (excluding esophageal resections and lung transplants). Our data thus are most likely representative of many centers in Europe. We observed a low overall rate of RBC transfusion of 7.4% in the entire cohort, with transfusion rates of 2.5% for elective thoracic procedures, 2.4% for lung resections, and 5.2% for lobectomy. These numbers are significantly lower than in several previous studies, where overall transfusion rates of 16.1% for thoracic procedures have been observed, with up to 23.6% for anatomic lung resections, up to 19.9% for lobectomy and up to 25.0% for pneumonectomy (8-11). Similarly, previously reported rates for wedge resections and chest-wall resections range between 25.0–40.0% but were significantly lower in our cohort (1.4% and 7.1%, respectively) (9). A more recent study by Latif et al. reported a rate of perioperative blood transfusion of 10.2% in patients undergoing anatomic lung resection for non-small cell lung cancer (5). Similarly Byrd et al. observed a transfusion rate of 8.5% in all cases with surgery for lung cancer within the STS database (16). A large single-center retrospective analysis by Abdelsattar et al. investigating a cohort of 6,280 patients found an overall transfusion rate of 7.1% (17). However, this study reported intraoperative blood transfusion only and included a different spectrum of surgical procedures and patients (47.7% esophageal operations, the majority of those for benign disease). Even with the low overall transfusion rate observed in this study, we still found high rates in specific subgroups, especially in urgent procedures (20.2%) and open surgery (20.0%). The highest transfusion rate was observed in surgery for empyema, where almost one in two patients required blood transfusion (44.7%), a nearly 10-fold higher risk when compared to lobectomy. This is not far from earlier published data where a transfusion rate of 60.0% in patients undergoing decortication was reported (9). In a recent STS database analysis, Towe et al. report an intra- and postoperative transfusion rate of 26.3% in a selected subset of acute empyema patients (parapneumonic empyema only, exclusion of patients with chronic empyema and malignancy) (18). As a note, we observed a relatively high transfusion rate (13.0%) for mediastinal procedures. This is plausible as this category consisted mainly of major procedures for mediastinal tumor resection or surgical management of septic complications of mediastinitis.
Current guidelines on hemotherapy and patient blood management advise a more restrictive transfusion policy than in the past. This may in part explain the lower transfusion rates in our cohort when compared to studies from the 1990s or 2000s. However, although several national and international guidelines exist, it is known that the practice of blood use differs widely between hospitals and departments, even today (19). The low transfusion rate in our cohort may therefore reflect adequate adherence to current hemotherapy guidelines, even if this cannot be proven by data provided here. The paradigm shift towards minimally invasive surgery like VATS and RATS in the past decade might also have contributed to a significant reduction in the need for blood transfusion. This is further indicated by the high rate of minimally invasive procedures in our study population (72.4%, including all non-elective operations). Moreover, the increasing implementation of enhanced recovery after surgery programs and the introduction of patient blood management protocols are likely to help limit the need for transfusion in elective thoracic surgery (20). The high transfusion rate in non-elective procedures—especially in patients with empyema—illustrates a shift in the need for blood products towards select high-risk patient populations.
To better identify patients at risk, and as is recommended by the recently updated STS guideline on patient blood management, we aimed to identify specific risk factors for a RBC transfusion event (12). Univariable and multivariable analysis revealed surgery for empyema, open surgery, low preoperative hemoglobin (<10.4 g/dL) and older patient age (>76.5 years) as the only independent risk factors for RBC transfusion in our thoracic surgery collective. These risk factors reflect those in previous studies on thoracic surgery and other surgical fields (10,21,22). When the general risk of transfusion is low but specific risk factors predicting a high transfusion probability in distinct patients can be identified, the still widespread use of maximum surgical blood ordering schedules, which specify the number of cross-matched units only based on the planned procedure, does seem less and less appropriate. Instead, it might be advisable to tailor the number of cross-matched units to the individual risk of the patient (21,23).
In our study, the crossmatch to transfusion ratio, the transfusion probability, and the transfusion index showed that the selectivity of our standard operating procedure regarding the risk of transfusion was poor and blood usage was inefficient. Therefore, it was most likely also not cost-effective, even if we did not perform a detailed cost analysis. Several authors have suggested ways to reduce the number of cross-matched units, to perform type and screen only, or to completely avoid type and screen in select low-risk patients (11,24-26). However, in this study, antibodies were detected in 7.6% of type and screen procedures. In these patients, the provision of cross-matched units would have been delayed in the absence of preoperative type and screen. At our institution, the blood bank is located outside the building complex with the operating theatres, which theoretically can lead to longer transport times for blood products. This consideration led to a high number of routine crossmatches due to patient safety considerations.
This study has several limitations. This was a single center retrospective analysis, which carries an inherent risk of bias and limits the validity of the findings. There may be unknown factors that were not assessed, and the study population was heterogeneous regarding the type of surgery performed. The number of patients in this study did not allow the analysis of all conceivable subgroups. The high rate of cross-matched patients in this study resulted from institution-specific requirements and cannot be generalized.
Conclusions
The rate of RBC transfusion in non-cardiac thoracic surgery has decreased significantly when compared to previous data, especially in elective lung resections including lobectomy. Transfusion rates remain high in open and urgent surgery, particularly in surgery for empyema. Preoperative ordering of RBC units should be tailored to patient-specific risk factors. Maximum surgical blood ordering based on the type of procedure alone seem outdated. For ethical and economic considerations, it might be advisable to handle preoperative ordering of blood more restrictively.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1581/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1581/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1581/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics review board approval (No. 2021-15979, date: 17.07.2021) was obtained from the ethics commission of the State Chamber of Physicians in Rhineland-Palatinate in Mainz, Germany. The review board waived the need for patient consent for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veseli B, Sandner S, Studte S, et al. The impact of COVID-19 on blood donations. PLoS One 2022;17:e0265171. [Crossref] [PubMed]
- Thomas P, Michelet P, Barlesi F, et al. Impact of blood transfusions on outcome after pneumonectomy for thoracic malignancies. Eur Respir J 2007;29:565-70. [Crossref] [PubMed]
- Ferraris VA, Davenport DL, Saha SP, et al. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having noncardiac thoracic operations. Ann Thorac Surg 2011;91:1674-80; discussion 1680. [Crossref] [PubMed]
- Nosotti M, Rebulla P, Riccardi D, et al. Correlation between perioperative blood transfusion and prognosis of patients subjected to surgery for stage I lung cancer. Chest 2003;124:102-7. [Crossref] [PubMed]
- Latif MJ, Tan KS, Molena D, et al. Perioperative blood transfusion has a dose-dependent relationship with disease recurrence and survival in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;157:2469-2477.e10. [Crossref] [PubMed]
- Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med 2017;377:2133-44. [Crossref] [PubMed]
- Shaw RE, Johnson CK, Ferrari G, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion 2014;54:1106-13. [Crossref] [PubMed]
- Gwin JL, Keller SM. Blood transfusion practices after resection of intrathoracic neoplasms. J Surg Oncol 1993;54:34-7. [Crossref] [PubMed]
- Griffiths EM, Kaplan DK, Goldstraw P, et al. Review of blood transfusion practices in thoracic surgery. Ann Thorac Surg 1994;57:736-9. [Crossref] [PubMed]
- Petrella F, Radice D, Randine MG, et al. Perioperative blood transfusion practices in oncologic thoracic surgery: when, why, and how. Ann Surg Oncol 2012;19:82-8. [Crossref] [PubMed]
- Devbhandari MP, Farid S, Goatman C, et al. Is type and screen only policy safe for patients undergoing elective lobectomy? Eur J Cardiothorac Surg 2013;44:1113-6; discussion 116. [Crossref] [PubMed]
- Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann Thorac Surg 2021;112:981-1004. [Crossref] [PubMed]
- Querschnitts-Leitlinien zur Therapie mit Blutkomponenten und Plasmaderivaten - Gesamtnovelle 2020. 5th ed. Deutscher Ärzteverlag, 2021. Available online: https://www.bundesaerztekammer.de/themen/medizin-und-ethik/wissenschaftlicher-beirat/stellungnahmen-richtlinien-jahresberichte/haemotherapie-transfusionsmedizin/querschnitts-leitlinien-baek-zur-therapie-mit-blutkomponenten-und-plasmaderivaten-gesamtnovelle-2020
- Abdelsattar ZM, Hendren S, Wong SL, et al. Variation in Transfusion Practices and the Effect on Outcomes After Noncardiac Surgery. Ann Surg 2015;262:1-6. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Byrd CT, Williams KM, Backhus LM. A brief overview of thoracic surgery in the United States. J Thorac Dis 2022;14:218-26. [Crossref] [PubMed]
- Abdelsattar ZM, Joshi V, Cassivi S, et al. Preoperative Type and Screen Before General Thoracic Surgery: A Nomogram to Reduce Unnecessary Tests. Ann Thorac Surg 2023;115:519-25. [Crossref] [PubMed]
- Towe CW, Carr SR, Donahue JM, et al. Morbidity and 30-day mortality after decortication for parapneumonic empyema and pleural effusion among patients in the Society of Thoracic Surgeons' General Thoracic Surgery Database. J Thorac Cardiovasc Surg 2019;157:1288-1297.e4. [Crossref] [PubMed]
- White MJ, Hazard SW 3rd, Frank SM, et al. The evolution of perioperative transfusion testing and blood ordering. Anesth Analg 2015;120:1196-203. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Stangenberg L, Curran T, Shuja F, et al. Development of a risk prediction model for transfusion in carotid endarterectomy and demonstration of cost-saving potential by avoidance of "type and screen". J Vasc Surg 2016;64:1711-8. [Crossref] [PubMed]
- Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396-407. [Crossref] [PubMed]
- Wang Z, Zhe S, Zimmerman J, et al. Development and validation of a machine learning method to predict intraoperative red blood cell transfusions in cardiothoracic surgery. Sci Rep 2022;12:1355. [Crossref] [PubMed]
- Boral LI, Henry JB. The type and screen: a safe alternative and supplement in selected surgical procedures. Transfusion 1977;17:163-8. [Crossref] [PubMed]
- Booth AT, Allen S, Simianu VV, et al. Selective type & screen for elective colectomy based on a transfusion risk score may generate substantial cost savings. Surg Endosc 2022;36:8817-24. [Crossref] [PubMed]
- van Klei WA, Moons KG, Leyssius AT, et al. A reduction in type and screen: preoperative prediction of RBC transfusions in surgery procedures with intermediate transfusion risks. Br J Anaesth 2001;87:250-7. [Crossref] [PubMed]




