
Anatomical spatial distribution of the bilateral coronary ostia and aortic valve commissures relative to the aortic arch
Highlight box
Key findings
• This study found a fixed angular relationship between the coronary ostia or aortic valve commissures and the IC of the aortic arch.
What is known and what is new?
• Previous studies have shown the importance of achieving commissural alignment during TAVR.
• This study found a fixed angular relationship between the coronary ostia or aortic valve commissures and the IC of the aortic arch.
What is the implication, and what should change now?
• This relationship could help to establish an individualized implantation method that would enable commissural and coronary alignment to be achieved in TAVR.
Introduction
Transcatheter aortic valve replacement (TAVR) is a recognized, minimally invasive technique for patients with severe aortic stenosis (AS). As TAVR technology has developed over the past decade, the indications for TAVR have been extended, compared to surgical aortic valve replacement (SAVR) (1,2), for use in younger patients, which has increased the life expectancy and prevalence of coronary artery diseases. Unlike SAVR, in which the surgical valve commissures can be completely aligned with the native valve commissures, commissural alignment during TAVR is not easily achieved, as the transcatheter heart valve (THV) is always implanted in a random axial direction (3). Previous studies have shown that the neo-commissural orientation of the THV affects coronary obstruction during TAVR, the long-term durability of the THV, and coronary artery access if re-interventions are required after TAVR (3-5). Commissural alignment also increases the feasibility of bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction in cases in which TAVR is performed due to failed THV implantation. Tang et al. found that the specific initial orientations of Evolut R/Pro (Medtronic, Minneapolis, Minnesota) and ACURATE Neo (Boston Scientific, MA, USA) aortic valves improve commissural alignment (6-8). Bilateral coronary ostia were observed under fluoroscopy during TAVR, and a certain anatomical relationship between the coronary arteries and the inner curve (IC) of the aortic arch was found. If confirmed, this relationship could help to establish an individualized implantation method that would enable commissural and coronary alignment to be achieved for THVs. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-482/rc).
Methods
Study population
Three-dimensional reconstruction was performed based on the pre-procedural computed tomography (CT) for 100 consecutive patients diagnosed with severe AS from July 2018 to August 2022 in The First Hospital of China Medical University. A total of 20 patients were excluded from the study because of the poor visualization of the coronary ostia and commissures or type 0 bicuspid aortic valves (BAVs), resulting in a final study population of 80 patients. Among the patients, 50 had tricuspid aortic valves (TAVs) and 30 had type 1 BAVs. The angles from the coronary ostia and the commissures of the aortic valve to the IC of the aortic arch were measured. Study approval was obtained from the institutional review board of The First Hospital of China Medical University (No. 2022QL103) and the study conformed to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) in terms of the principles for medical research involving human subjects. All the subjects had given written informed consent to participate in the study.
CT image acquisition and analysis
All the patients underwent pre-procedural electrocardiographically gated CT angiography with a second-generation dual-source CT scanner (Somatom Definition Flash; Siemens Healthcare, Germany). The angles between the bilateral coronary ostia or aortic valve commissures and IC were analyzed using FluoroCT software (Circle Cardiovascular Imaging Inc., Calgary, Canada).
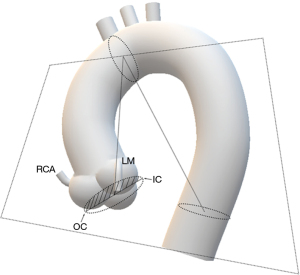
Definition of IC
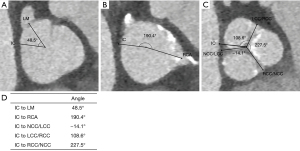
Three center points of the aortic annulus, the aortic arch at the level of the brachiocephalic trunk, and the descending aorta parallel to the aortic annulus were selected. The arch plane was then defined by the three center points. The two intersection points of the annulus with this plane were the IC and outer curve of the annulus relative to the aortic arch, respectively (see Figure 1). The angles from the IC to the left main (LM), from the IC to the right coronary artery (RCA), from the IC to the non-coronary cusp (NCC)/left coronary cusp (LCC) commissure, from the IC to the LCC/right coronary cusp (RCC) commissure, and from the IC to the RCC/NCC commissure were measured for every patient (see Figure 2).
Statistical analysis
The continuous variables are presented as the mean ± standard deviation or the median with the interquartile range (IQR) for those with normal or skewed distributions, respectively. The categorical variables are presented as the proportion. All the analyses were performed using SPSS 26.0 software (IBM, Armonk, NY, USA).
Results
Baseline characteristics
The baseline characteristics of the patients are listed in Table 1. The patients had a mean age of 74.9±8.8 years, and 43.8% of the patients were female. Most of the patients (52.5%) were classified in the New York Heart Association class III or IV at admission, with a median Society of Thoracic Surgeons score of 4.5% with an IQR of 2.6–6.7%. The median left ventricular ejection fraction was 50.0% with an IQR of 38.0–63.0%, with a mean gradient of 54.3±16.6 mmHg, and 28 patients (35.0%) had at least moderate aortic regurgitation. In addition, 50 patients (62.5%) had TAVs and 30 patients (37.5%) had type 1 BAVs.
Table 1
| Variables | N=80 |
|---|---|
| Age (years) | 74.9±8.8 |
| Female sex | 35 (43.8) |
| BMI (kg/m2) | 23.3±3.4 |
| Atrial fibrillation | 20 (25.0) |
| Hypertension | 41 (51.2) |
| Diabetes | 17 (21.3) |
| Chronic kidney disease | 19 (23.8) |
| COPD | 11 (13.8) |
| Prior PCI | 9 (11.3) |
| Prior CABG | 0 (0.0) |
| Prior stroke | 7 (8.8) |
| Prior permanent pacemaker | 1 (1.3) |
| STS score (%) | 4.5 (2.6–6.7) |
| NYHA (class III–IV) | 42 (52.5) |
| LVEF (%) | 50.0 (38.0–63.0) |
| Mean gradient (mmHg) | 54.3±16.6 |
| AR ≥ moderate | 28 (35.0) |
| TAV | 50 (62.5) |
| Type 1 BAV | 30 (37.5) |
Values are the mean ± standard deviation, n (%), or median (range). BMI, body mass index; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; STS, Society of Thoracic Surgeons; NYHA, New York Heart Association; LVEF, left ventricle ejection fraction; AR, aortic regurgitation; TAV, tricuspid aortic valve; BAV, bicuspid aortic valve.
Anatomy of the bilateral coronary ostia and aortic valve commissures relative to the aortic arch
The pre-procedural CT analysis of all the patients showed the special anatomical characteristics of the bilateral coronary ostia and aortic valve commissures relative to the aortic arch (see Figure 3). The angles from the bilateral coronary ostia and aortic valve commissures to the IC of each case are shown in Figure S1. For the bilateral coronary ostia, the mean angle from the IC to the LM was 48.0°±17.5°, the mean angle from the IC to the RCA was 172.6°±15.2°, and the mean angle from the LM to the RCA was 124.6°±19.9° (see Table 2).
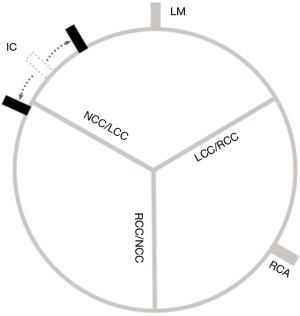
Table 2
| Variables | TAVs (n=50) | Type 1 BAVs (n=30) | Total (n=80) |
|---|---|---|---|
| IC to LM (°) | 42.8±13.6 | 56.8±19.8 | 48.0±17.5 |
| IC to RCA (°) | 173.6±16.0 | 171.0±13.9 | 172.6±15.2 |
| LM to RCA (°) | 130.8±16.9 | 114.2±20.4 | 124.6±19.9 |
| IC to NCC/LCC (°) | −16.2±13.2 | −1.5±23.5 | −12.8 (−21.5 to −2.2) |
| IC to LCC/RCC (°) | 99.4±13.0 | 107.4±17.2 | 102.4±15.1 |
| IC to RCC/NCC (°) | 219.6±13.0 | 220.2±15.5 | 219.9±13.9 |
Values are the mean ± SD or median (range). TAV, tricuspid aortic valve; BAV, bicuspid aortic valve; IC, inner curve; LM, left main; RCA, right coronary artery; NCC, non-coronary cusp; LCC, left coronary cusp; RCC, right coronary cusp; SD, standard deviation.
For the 50 cases with TAVs, the mean angle from the IC to the LM was 42.8°±13.6°, the mean angle from the IC to the RCA was 173.6°±16.0°, and the mean angle from the LM to the RCA was 130.8°±16.9°. For the 30 cases with type 1 BAVs, the mean angle from the IC to the LM was 56.8°±19.8°, the mean angle from the IC to the RCA was 171.0°±13.9°, and the mean angle from the LM to the RCA was 114.2°±20.4°.
For the aortic valve commissures, the median angle from the IC to the NCC/LCC was −12.8° with an IQR of −21.5° to −2.2°, the mean angle from the IC to the LCC/RCC was 102.4°±15.1°, and the mean angle from the IC to the RCC/NCC was 219.9°±13.9°.
For the 50 cases with TAVs, the angle was more accurate. Specifically, the mean angle from the IC to the NCC/LCC was −16.2°±13.2°, the mean angle from the IC to the LCC/RCC was 99.4°±13.0°, and the mean angle from the IC to the RCC/NCC was 219.6°±13.0°.
The main error occurred in the 30 patients with type 1 BAVs, in which the mean angle from the IC to the NCC/LCC was −1.5°±23.5°, the mean angle from the IC to the LCC/RCC was 107.4°±17.2°, and the mean angle from the IC to the RCC/NCC was 220.2°±15.5°.
Discussion
This study found a fixed angular relationship between the LM and IC, with a mean angle of 48.0°±17.5°. Given the cusp asymmetry and coronary ostial eccentricity found by Wang et al. (9), the angle from the IC to the RCA was also measured, and the mean angle was 172.6°±15.2°. The mean angle from the LM to the RCA was 124.6°±19.9°, which was close to 120°. There was no obvious difference between the TAVs and type 1 BAVs. This made more sense for the anatomical relationship between the aortic valve commissure and IC; however, due to the asymmetrical structure of the type 1 BAVs, it would be helpful for commissural alignment during TAVR to analyze patients with TAVs alone.
Given this special anatomy, the neo-commissural orientation of THVs could be adjusted in the descending aorta to the corresponding angle as measured and then passed through the aortic arch to the ascending aorta to achieve better coronary alignment. By using this method to accurately measure the angle from the IC to the coronary artery or aortic valve commissures for every case before the procedure, the implantation method of THVs could be more individualized.
Tang et al. found that the specific initial orientations of the self-expanding aortic valves could improve commissural alignment (6-8). For the Evolut R/Pro aortic valves, commissural alignment was achieved by inserting the delivery catheter with the flush port facing the 3 o’clock position, while for the ACURATE Neo valves, commissural alignment was achieved by inserting the delivery catheter with the flush port facing the 12 o’clock position. The main mechanism underlying this phenomenon is that the anatomical location of the coronary arteries and aortic valve commissures is fixed. The second mechanism may be related to the spine location within the delivery system, which limits any significant rotation, as it tracks from the descending aorta to the annulus (7).
If the manufacturers designed the orientation of THVs in the delivery system with this data as a benchmark, a more precise method for achieving commissural and coronary alignment could be established, and patient prognosis after the procedure could be significantly improved.
Study limitations
This was a single-center retrospective study with a small number of patients. Future studies with larger sample sizes are needed.
Conclusions
There are anatomical relationships between the coronary ostia or aortic valve commissures and the IC of the aortic arch. This relationship could help to establish an individualized implantation method suitable for THVs to achieve commissural and coronary alignment.
Acknowledgments
We would like to thank International Science Editing (https://www.internationalscienceediting.com) for editing this manuscript.
Funding: This work was supported by the Shenyang Science and Technology Program (No. 22-321-33-09).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-482/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-482/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-482/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-482/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Study approval was obtained from the institutional review board of The First Hospital of China Medical University (No. 2022QL103) and the study conformed to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) in terms of the principles for medical research involving human subjects. All the subjects gave written informed consent to participate in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Writing Committee Members. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25-e197. [Crossref] [PubMed]
- Fuchs A, Kofoed KF, Yoon SH, et al. Commissural Alignment of Bioprosthetic Aortic Valve and Native Aortic Valve Following Surgical and Transcatheter Aortic Valve Replacement and its Impact on Valvular Function and Coronary Filling. JACC Cardiovasc Interv 2018;11:1733-43. [Crossref] [PubMed]
- Ochiai T, Chakravarty T, Yoon SH, et al. Coronary Access After TAVR. JACC Cardiovasc Interv 2020;13:693-705. [Crossref] [PubMed]
- Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv 2019;12:1197-216. [Crossref] [PubMed]
- Tang GHL, Zaid S, Gupta E, et al. Impact of Initial Evolut Transcatheter Aortic Valve Replacement Deployment Orientation on Final Valve Orientation and Coronary Reaccess. Circ Cardiovasc Interv 2019;12:e008044. [Crossref] [PubMed]
- Tang GHL, Zaid S, Fuchs A, et al. Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR): Impact on Final Valve Orientation and Coronary Artery Overlap. JACC Cardiovasc Interv 2020;13:1030-42. [Crossref] [PubMed]
- Tang GHL, Sengupta A, Alexis SL, et al. Conventional versus modified delivery system technique in commissural alignment from the Evolut low-risk CT substudy. Catheter Cardiovasc Interv 2022;99:924-31. [Crossref] [PubMed]
- Wang X, De Backer O, Bieliauskas G, et al. Cusp Symmetry and Coronary Ostial Eccentricity and its Impact on Coronary Access Following TAVR. JACC Cardiovasc Interv 2022;15:123-34. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


