
Diagnostic sensitivity of solid volume measurement for pathological invasion in non-solid lung adenocarcinoma
Highlight box
Key findings
• TS and SS, not 3D parameters were significantly associated with IAD, although sensitivity of SV was higher than that of SS in the diagnosis of IAD with a TS of 2.1–3.0 cm.
What is known and what is new?
• The discrepancy between SS defined by the current TNM classification and IS has existed practically.
• Eleven out of 18 IAD cases (61.1%) with SS ≤5 mm were diagnosed accurately by using SV.
What is the implication, and what should change now?
• SV measurement may compensate for the sensitivity of the current cT diagnosis to diagnose IAD in radiological adenocarcinoma sized 2.1–3.0 cm.
Introduction
Background
A variety of small-sized lung cancer images can be obtained using the novel innovations of high resolution-computed tomography (HR-CT). Impacts of ground-glass opacity (GGO) lesions on survival outcomes have been evaluated in recent decades. Several factors besides radiological total size (TS), including tumor disappearance rate, consolidation tumor ratio (CTR), and solid size (SS), have been suggested as potential factors that predict lung cancer outcomes (1-4). The Japan Clinical Oncology Group (JCOG) launched clinical trials of limited resections for early-stage lung cancer using CTR criteria based on a JCOG 0201 study (5). Limited impact of GGO and the corresponding lepidic lesion on survival were widely reported in lung adenocarcinoma; SS has been focused on as one of the promising prognostic factors. Subsequently, the clinical T descriptor has been defined by SS, although it had been defined by TS previously. The current 8th tumor-node-metastasis (TNM) classification defines a lesion with SS greater than 5 mm radiologically diagnosed as invasive adenocarcinoma (IAD) (6).
Rationale and knowledge gap
Although the survival outcomes of patients who are pathologically diagnosed with adenocarcinoma in situ (AIS) or minimally IAD (MIA) are known to be extremely favorable, the discrepancy between SS and pathological invasive size (IS) practically exists in the clinics (7,8). One of the potential reasons for discrepancies is the bias induced by the physicians who measure SS. Although HR-CT slice is advantageous in terms of easy measurement, diagnoses of solid parts depend strongly on the physician’s subjectivity unlike for the diagnoses of pathological invasive findings. To predict the biological nature of small-sized adenocarcinomas and explore novel surgical strategies, estimating the pathological invasion appropriately before surgery is crucial.
Objective
In the present study, we semi-automatically measured three-dimensional (3D) parameters including the solid volume (SV) using a volume analyzing application, which does not require the specific CT slice, and investigated the association between those parameters and IAD. We also evaluated the ability of 3D parameters to diagnose pathologically invasive small-sized adenocarcinoma and compared this method with SS. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1603/rc).
Methods
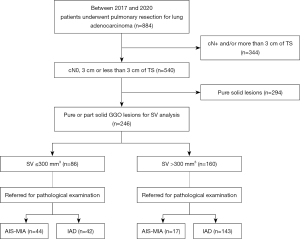
Between January 2017 and December 2020, 884 consecutive patients underwent pulmonary resection for lung adenocarcinoma at Shizuoka Cancer Center. Among them, patients with cN0 tumor radiologically sized ≤3 cm and pure or part solid GGO lesion were eligible for the study. Two hundred ninety-four patients with pure solid nodules were excluded due to the potential aggressive characteristics of these nodules for which limited resection was not known to preserve prognosis (9,10). The remaining 246 patients with non-solid nodules were enrolled in this study (Figure 1). Of them, 173 (70.3%), 35 (14.2%), and 38 (15.5%) patients underwent lobectomy, segmentectomy, and wedge resection, respectively. Clinicopathological data, such as age, gender, smoking history, pre-operative serum carcinoembryonic antigen (CEA) level, TS, SS, CTR, pathological whole tumor size, IS, pN status, and presence of lymphatic invasion (ly), vascular invasion (v), and pleural invasion (pl) were reviewed from the medical records. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective diagnostic study was approved by the institutional review board of Shizuoka Cancer Center (No. J2020-1-2020-1-3) and was not registered. The need for obtaining written informed consent from patients was waived due to the retrospective nature of this study.
All histologic sections of completely submitted tumor were stained with hematoxylin and eosin and were reviewed by two pathologists. Whole tumor size and IS were determined by direct measurement. In addition, all cases were evaluated for the presence of ly, v, and pl.
CT scan was performed using a multi-detector CT scanner (Aquilion16; Toshiba Medical Systems Corporation, Tochigi, Japan). Images with a slice thickness of 1–2 mm were acquired in the helical mode without intravascular contrast material. SS measurement was performed and confirmed by our surgical cancer board. Digital images were transferred to a teleradiology workstation, and volume-rendered 3D models of the lungs were retrospectively reconstructed using the Synapse Vincent imaging application (FUJIFILM, Tokyo, Japan). This application enabled the measurement of 3D data semi-automatically without selecting a specific slice; 3D data of max Hounsfield unit (HU), mean HU, a total volume of more than −800 HU, and SV of more than −300 HU were measured in the targeted lesion (Figure 2). This software algorithm was used in previous reports (11,12). To evaluate the diagnostic ability of 3D parameters in diagnosing IAD, receiver operating characteristic (ROC) curves were plotted for max HU, mean HU, and SV. Cut-off values were set for respective variables and used for further analyses. Diagnostic abilities of SS (>5 mm), which has been defined in the 8th TNM classification, and the set cut-off value for SV were compared. To explore differences of diagnostic abilities by TS, 0–2.0 cm, and 2.1–3.0 cm of the TS tumor was compared, in addition to the whole cohort. Furthermore, investigations for diagnostic ability in IAD with either ly, v, pl or nodal metastases, which is termed aggressive IAD, were carried out.

Statistical analysis
Statistical analyses were performed using SPSS software for Windows, version 12.0 (IBM, Armonk, NY, USA). Comparison of patient categorical variables between the groups and area under ROC curves between respective parameters were performed using the chi-squared test. Continuous variables were compared using Mann-Whitney U test. Univariate and multivariate logistic regression analyses were performed to identify factors predicting IAD, with P<0.05 indicating statistical significance. Confounding factors were adjusted by the multivariate analysis.
Results
Demographics of enroled 246 patients are shown in Table 1. Approximately 10% of the patients presented with a high serum CEA level ≥5 ng/mL before pulmonary resection. Median TS and SS were 20 mm and 8 mm, respectively. Altogether, 152 patients (61.8%) had nodules with >5 mm of SS. The median pathological whole tumor size and IS were 18 and 10 mm, respectively. Overall, 183 patients (74.4%) had IAD, 51 patients (20.7%) had MIA, and 12 patients (4.9%) had AIS. In larger TS tumor of 2.1–3.0 cm, TS, SS, max HU, and SV were significantly higher compared to those of the smaller tumor. Further, more patients with radiological IAD (SS >5 mm) were included significantly. Other pathological invasive findings, such as ly, v, and pl, were diagnosed in limited numbers of patients. Pathological lymph node metastases were diagnosed in 2 cases (0.8%) (Table 2). Twenty-three aggressive IAD cases were identified. Among 246 patients, 4 patients experienced recurrence, 7 patients died of other causes. No patients died of lung cancer.
Table 1
| Variables | Total (n=246) | 0–2.0 cm (n=127) | 2.1–3.0 cm (n=119) | P value |
|---|---|---|---|---|
| Age >75 years old, n (%) | 67 (27.2) | 35 (27.6) | 32 (26.9) | >0.99 |
| Male, n (%) | 99 (40.2) | 52 (40.9) | 47 (39.5) | 0.90 |
| Never smoker, n (%) | 128 (52.0) | 64 (50.4) | 64 (53.8) | 0.61 |
| Pre.Op CEA ≥5 ng/mL, n (%) | 24 (9.8) | 12 (9.4) | 12 (10.1) | >0.99 |
| TS (mm), median [IQR] | 20 [16–25] | 17 [14–18] | 25 [22–27] | <0.001 |
| SS (mm), median [IQR] | 8 [4–13] | 5 [3–9] | 12 [6–17] | <0.001 |
| SS >5 mm, n (%) | 152 (61.8) | 63 (49.6) | 92 (77.3) | <0.001 |
| CTR >0.5, n (%) | 97 (39.4) | 43 (33.9) | 54 (45.4) | 0.07 |
| Max HU, median [IQR] | 381 [311–458] | 349 [260–405] | 415 [352–515] | <0.001 |
| Mean HU, median [IQR] | −522 [−641 to −410] | −538 [−657 to −446] | −505 [−628 to −356] | 0.05 |
| SV (mm3), median [IQR] | 514 [217–1,085] | 241 [107–463] | 983 [593–1,799] | <0.001 |
P value represent the result of statistical tests of two groups stratified by size. TS, total size; Pre.Op, preoperative; CEA, carcinoembryonic antigen; IQR, interquartile range; SS, solid size; CTR, consolidation tumor ratio; HU, Hounsfield unit; SV, solid volume.
Table 2
| Variables | Total (n=246) | 0–2.0 cm (n=127) | 2.1–3.0 cm (n=119) | P value |
|---|---|---|---|---|
| Whole tumor size (mm), median [IQR] | 18 [14–22] | 15 [12–17] | 22 [20–25] | <0.001 |
| IS (mm), median [IQR] | 10 [5–15] | 7 [4–11] | 15 [10–19] | <0.001 |
| IS >5 mm (IAD), n (%) | 183 (74.4) | 76 (59.8) | 107 (89.9) | <0.001 |
| pl+, n (%) | 12 (4.8) | 0 (0.0) | 11 (9.2) | 0.004 |
| ly+, n (%) | 9 (3.7) | 2 (1.6) | 7 (5.9) | 0.09 |
| v+, n (%) | 5 (2.0) | 2 (1.6) | 3 (2.5) | 0.67 |
| pN+, n (%) | 2 (0.8) | 0 (0.0) | 2 (1.7) | 0.23 |
P value represent the result of statistical tests of two groups stratified by size. TS, total size; IQR, interquartile range; IS, invasive size; IAD, invasive adenocarcinoma; pl, pleural invasion; ly, lymphatic invasion; v, vascular invasion; pN, pathological nodal metastasis.
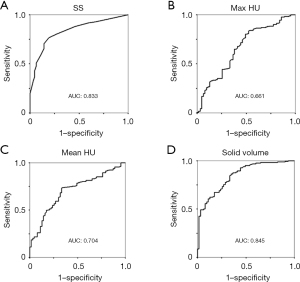
In 3D data measurement, the median max HU, median HU and median SV were 381, −522, and 514 mm3, respectively (Table 1). ROC curves were generated to evaluate the diagnostic abilities of SS, max HU, mean HU, and SV for IAD. Areas under the curve (AUC) for SS and SV were 0.83 and 0.84, respectively, significantly higher than those of max HU and mean HU. The difference between the AUCs of SS and SV was not significant (P=0.77) (Figure 3). Furthermore, according to the ROC curves, the cut-off values were set as 5 mm, 320, −600, and 300 mm3 for SS, max HU, mean HU, and SV, respectively. Among 160 patients with SV >300 mm3, 143 patients (89.3%) were diagnosed with IAD, meanwhile 42 patients (48.8%) out of 86 patients with SV ≤300 mm3 were diagnosed with IAD (Figure 1).
In univariate analyses, TS >20 mm, SS >5 mm, CTR >0.5, max HU >320, mean HU >−600, and SV >300 mm3 were significantly associated with the occurrence of IAD. Multivariate analysis revealed that TS and SS were significantly associated with IAD, whereas variables derived from 3D data were not (Table 3).
Table 3
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| TS >20 mm | 5.984 | 2.989–11.98 | <0.001 | 3.496 | 1.442–8.476 | 0.006 | |
| SS >5 mm | 11.11 | 4.879–25.33 | <0.001 | 5.984 | 2.167–16.52 | 0.001 | |
| CTR >0.5 | 7.741 | 3.350–17.88 | <0.001 | 1.168 | 0.342–3.990 | 0.80 | |
| HU max >320 | 4.215 | 2.281–7.786 | <0.001 | 2.064 | 0.927–4.597 | 0.08 | |
| HU mean >−600 | 5.393 | 2.915–9.975 | <0.001 | 2.164 | 0.921–5.082 | 0.08 | |
| SV >300 mm3 | 8.659 | 4.531–16.55 | <0.001 | 1.168 | 0.342–3.990 | 0.80 | |
IAD, invasive adenocarcinoma; OR, odds ratio; CI, confidence interval; TS, total tumor size; SS, solid size; CTR, consolidation tumor ratio; HU, Hounsfield unit; SV, solid volume.
The cross tabulations of SS against IS and SV against IS were presented as Tables S1,S2, diagnostic abilities of SS and SV with cut-off values of 5 mm and 300 mm3, respectively, in predicting IAD are presented in Table 4. In the whole cohort, with nodules measuring ≤3 cm, sensitivities and accuracies were similar for both the parameters. The sensitivity of SV was lower (0.55) than that of SS (0.67) in tumors measuring ≤2 cm but higher in tumors >2 cm. Eleven out of 18 IAD cases (61.1%) with SS ≤5 mm were diagnosed accurately by SV. Furthermore, the diagnostic abilities of SS and SV with cut-off values of 12 mm and 655 mm3 being set based on ROC curves, respectively, in predicting aggressive IAD are presented in Table S3. Sensitivities of SS and SV were similar. No adverse events were reported during this retrospective diagnostic study.
Table 4
| TS | Parameters | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| Total | SS | 0.76 | 0.82 | 0.93 | 0.53 | 0.77 |
| SV | 0.77 | 0.72 | 0.89 | 0.51 | 0.76 | |
| 0–2.0 cm | SS | 0.67 | 0.82 | 0.85 | 0.61 | 0.72 |
| SV | 0.55 | 0.82 | 0.83 | 0.53 | 0.65 | |
| 2.1–3.0 cm | SS | 0.83 | 0.83 | 0.98 | 0.36 | 0.83 |
| SV | 0.93 | 0.33 | 0.93 | 0.36 | 0.87 |
SS, solid size; SV, solid volume; IAD, invasive adenocarcinoma; TS, total size; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Key findings
We finally demonstrated that TS and SS, not 3D parameters were significantly associated with IAD in the whole cohort, although we documented higher sensitivity of SV in the diagnosis of IAD with a TS of 2.1–3.0 cm. Sensitivities of SS and SV for IAD were similar (0.76 and 0.77, respectively). And 44 of 94 cTis-T1mi lesions (45.7%) were diagnosed as IAD after surgery. Among those 44 cases, we observed only one patient with recurrence and two patients with aggressive IAD; most false negative cases might be less aggressive IAD with favorable prognosis (13).
Strengths and limitations
In the present study, we focused on excluding potential biases in patient selection and measurement in the CT images by using 3D measurement. The measurements were semi-automatically performed using a volume analyzing application after setting the region of interest before analyses. Although we did not observe a superior sensitivity of SV to SS for IAD diagnosis, SV sensitively detected 11 (61.1%) out of 18 IAD cases missed by SS.
Since our study was conducted in a single center and was retrospective in nature, it had several limitations. First, our study cannot exclude potential bias completely. We enrolled patients who were indicated for pulmonary resection; therefore, the number of patients with less aggressive adenocarcinoma of smaller SS and AIS/MIA were limited. Large series studies were required to evaluate diagnostic abilities in less aggressive adenocarcinoma furthermore and to validate our findings. Second, the lack of objective pathology review may be another limitation. In the present study, we focused on the issue about subjectivity in SS measurement and explore the potential usefulness of 3D measurement. Subjectivity by pathologists in measurement of IS might be another issue. However, Thunnissen et al. had reported fair reproducibility distinguishing invasive from in-situ tumors (14), and Boland et al. reported that good agreement was present between observers when classifying tumor as AIS, MIA, and IAD (15). According their reports, impact of this issue may not be so significant. However, there is a limitation in 3D measurement by the application itself; the application cannot discriminate anatomical structures from solid part of the tumor. Especially, centrally located lesions, compared with peripheral lesions, tend to be more affected by this problem, and SV of central lesions can be overestimated.
Comparison with similar researches
Earlier this century, researchers had focused on GGO, its corresponding lepidic proliferative components, and survival outcomes. Kodama et al. reported extremely favorable relapse-free survival rates of patients with GGO >50% (1). Matsuguma et al. also reported that a proportion of GGO can predict the tumor aggressiveness of nodal involvement, lymphovascular invasion, and histological subtype (2). Ohde et al. reported that CTR <0.5 could diagnose the least invasive disease, which had neither nodal nor lymphovascular involvement, with 100% specificity (3). To confirm these retrospective findings, JCOG tentatively defined radiological non-invasive lung cancer as a tumor with CTR <0.5 and evaluated its abilities to diagnose pathological non-invasive lung cancer (JCOG 0201 study) (5). Although this study did not meet the primary endpoint, the exploratory analysis revealed a 98.7% specificity with a cut-off value of 0.25 in tumors measuring ≤2 cm. Furthermore, according to the subsequent survival outcomes, a cut-off value of 0.5 was suggested to diagnose radiological non-IAD measuring 2–3 cm (16); thereafter, several limited resection trials have been carried out using these criteria (17-20). In 2011, the International Association for the study of Lung Cancer/American Thoracic Society/European Respiratory Society proposed an international multidisciplinary classification of lung adenocarcinoma (21). According to the new concepts, AIS and MIA with IS ≤5 mm will have approximately 100% disease-specific survival after complete resection. This proposal was validated by researchers (22-24). The 8th TNM classification of malignant tumors has defined the clinical T descriptor using SS and the pathological one using IS (6). Therefore, discrimination of IAD from AIS or MIA by pre-operative estimation of 5 mm IS has become more important. Lee et al. documented a strong correlation between the SS on CT and invasive component on pathology in the lung window setting (25). Sakao et al. (26), Sakakura et al. (7), and Samejima et al. (8) reported the usefulness of predicting IS using the tumor diameter in the mediastinal window setting. The diagnostic abilities of SS >5 mm for IAD varied among reports. Roberts et al. documented a 59% sensitivity (27), whereas higher sensitivities of 89–95.4% were also reported (7,25). Many studies have discussed the indications of limited resections in small-sized lung cancers, the discrepancy between cT and pT status has not been resolved to date. Some studies reported the potential usefulness of 3D-CT, however, those results are inconsistent. Kitazawa et al. reported that a mean CT value of −489 HU could predict IAD in ground-glass lung nodules sized <20 mm (28). Shimada et al. documented a significant association between SV and overall survival and disease-free survival in clinical stage I (TNM 8th), wherein enrollment was based on SS (12). Kawaguchi et al. also evaluated the usefulness of SV in predicting IAD in clinical stage IA (TNM 8th) adenocarcinoma including pure solid nodules (11); however, similar to our results, their findings failed to demonstrate the superior diagnostic abilities of SV over SS.
Explanations of findings
In the present study, we did not reveal significant associations between 3D parameters and IAD diagnosed based on pathological IS. These results may be attributed to IS being a one-dimensional parameter based on the selection of a specific section by pathologists. SS, measured on specific HR-CT slices by physicians, but not the SV measured semi-automatically, may correlate well with it. Another reason is the underlying discrepancy between the radiological solid portion and invasion. As we described above SV may contain the anatomical structures of the vessels and bronchus, especially in the central area. Moreover, collapse of the lung parenchyma and fibrotic lesions might also present a solid pattern. These limitations in measurement might have influenced our results.
Implications and actions needed
Herein, we presented the differences in the sensitivities of SS and SV, according to TS, in the diagnosis of IAD. It is presumed that SS tends to underestimate the IS in 2.1–3.0 TS tumors compared to that in the smaller ones. Therefore, SS may be less useful in predicting IS in large tumors.
Conclusions
The diagnosis of IAD was closely associated with one-dimensional pathological measurement, such as SS and TS, but not with 3D parameters, including SV. SV measurement may compensate for the sensitivity of the current cT diagnosis to predict IAD in radiological adenocarcinoma sized 2.1–3.0 cm.
Acknowledgments
The authors would like to thank Editage for the English language review.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1603/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1603/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1603/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1603/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Shizuoka Cancer Center (No. J2020-1-2020-1-3). The need for obtaining written informed consent from each patient was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [Crossref] [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [Crossref] [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Discrepancy of computed tomographic image between lung and mediastinal windows as a prognostic implication in small lung adenocarcinoma. Ann Thorac Surg 2003;76:1828-32; discussion 1832. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Sakakura N, Inaba Y, Yatabe Y, et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: A consideration based on lung and mediastinal window settings. Lung Cancer 2016;95:51-6. [Crossref] [PubMed]
- Samejima J, Ito H, Nakayama H, et al. Which Window Setting Is Best for Estimating Pathologic Invasive Size and Invasiveness? Ann Thorac Surg 2019;108:384-91. [Crossref] [PubMed]
- Aokage K, Miyoshi T, Ishii G, et al. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:533-42. [Crossref] [PubMed]
- Hattori A, Hirayama S, Matsunaga T, et al. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2019;14:265-75. [Crossref] [PubMed]
- Kawaguchi Y, Nakao M, Omura K, et al. The utility of three-dimensional computed tomography for prediction of tumor invasiveness in clinical stage IA lung adenocarcinoma. J Thorac Dis 2020;12:7218-26. [Crossref] [PubMed]
- Shimada Y, Furumoto H, Imai K, et al. Prognostic value of tumor solid-part size and solid-part volume in patients with clinical stage I non-small cell lung cancer. J Thorac Dis 2018;10:6491-500. [Crossref] [PubMed]
- Yambayev I, Sullivan TB, Suzuki K, et al. Pulmonary Adenocarcinomas of Low Malignant Potential: Proposed Criteria to Expand the Spectrum Beyond Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma. Am J Surg Pathol 2021;45:567-76. [Crossref] [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [Crossref] [PubMed]
- Boland JM, Froemming AT, Wampfler JA, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol 2016;51:41-50. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 2014;9:74-82. [Crossref] [PubMed]
- Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014;9:e110305. [Crossref] [PubMed]
- Roberts JM, Greenlaw K, English JC, et al. Radiological-pathological correlation of subsolid pulmonary nodules: A single centre retrospective evaluation of the 2011 IASLC adenocarcinoma classification system. Lung Cancer 2020;147:39-44. [Crossref] [PubMed]
- Kitazawa S, Saeki Y, Kobayashi N, et al. Three-dimensional mean CT attenuation value of pure and part-solid ground-glass lung nodules may predict invasiveness in early adenocarcinoma. Clin Radiol 2019;74:944-9. [Crossref] [PubMed]


