Safety and feasibility of prolonged bronchoscopy involving diagnosis of lung cancer, systematic nodal staging, and fiducial marker placement in a high-risk population
Introduction
Approximately twenty five percent of patients with Stage I NSCLC are not eligible for surgery due to medical comorbidities or advanced age. Stereotactic body radiation therapy (SBRT) is now considered the standard treatment for this population having shown similar control rates as surgery (1). Furthermore, there are recent reports suggesting that SBRT could be an option for treating operable patients with Stage I NSCLC (2). A potential disadvantage of SBRT is the lack of mediastinal sampling when compared with surgery. Another potential drawback is the need of fiducial markers (FMs) by some SBRT systems and for smaller tumors or tumors obscured by the spine. This need has been associated with treatment delays and complications such as pneumothorax, particularly when FMs are placed with CT-guidance (3-6).
Bronchoscopy has been proven to be an effective means for diagnosis of peripheral lung tumors (guided-bronchoscopy) (7), it is recommended as the initial choice for invasive staging of the mediastinum with endobronchial ultrasound (EBUS-) (8), and it has been proven to be successful and safe in delivering FMs to the vicinity of the tumors (9-16). In patients with a lung nodule and suspicion for early lung cancer who are considered medically inoperable, bronchoscopy could potentially provide diagnosis of lung cancer, nodal staging, and deployment of FMs for SBRT in a single bronchoscopic procedure, thus expediting patient care and preventing complications of CT-guided procedures. This would require the use of rapid on-site cytology examination (ROSE) since the diagnosis of lung cancer needs to be corroborated during the procedure and multiple mediastinal and hilar lymph nodes (LN) need to be assessed before making the decision to deploy FMs. The combination of these three procedures including peripheral bronchoscopy for diagnosis, systematic mediastinal and hilar staging (patients need to be N0 to be candidates for SBRT), and FM placement can result in prolonged bronchoscopic procedures. Of note, the same comorbidities that preclude these patients from having thoracic surgery also make them high-risk for complications in prolonged bronchoscopic procedures that are typically performed using general anesthesia.
The aim of this study is to describe -in terms of safety and feasibility- a cohort of patients who were suspected to have early stage lung cancer, were considered medically inoperable, and underwent bronchoscopy for diagnosis, nodal staging and placement of FMs in a single procedure.
Methods
This descriptive evaluation of a retrospective cohort was performed in a single center and received ethics approval by the local Institutional Review Board of Baylor College of Medicine (protocol number: H-35007). The charts of adult patients who underwent bronchoscopy at the Michael E. DeBakey VA Medical Center between January 2011 and July 2015 were reviewed. Patients met criteria for our study when their bronchoscopy involved the following 3 procedures in a single setting: initial diagnosis of lung cancer, systematic nodal staging with EBUS-TBNA, and placement of FMs. The main goal of this study was to evaluate the feasibility and safety of these three procedures in a single bronchoscopy. Procedures were considered successful when diagnosis was achieved, staging was fulfilled, FMs were placed, and the patient subsequently underwent SBRT without the need for additional markers. Baseline demographic and clinical variables including age, sex, BMI, ASA classification, ECOG performance status, smoking history, medical comorbidities (including obstructive sleep apnea, cardiovascular disease, and pulmonary disease), and pulmonary function test (when available) were recorded. Both procedure-related and anesthesia-related complications, as well as the need for escalation of care were recorded.
Procedure technique
Prior to bronchoscopy, patients were evaluated by a multidisciplinary panel, which considered them medically inoperable and adequate candidates for SBRT in case of lung cancer and N0 disease. All procedures were performed at the bronchoscopy lab under general anesthesia with a laryngeal mask airway (LMA). Procedure time was recorded from insertion of first bronchoscope to removal of last utilized bronchoscope. ROSE was present in all cases to determine both diagnosis of peripheral lung lesion as well as N stage by EBUS-TBNA. FMs were only placed if all LN were negative for malignancy and diagnosis of cancer was confirmed or there was a high suspicion for lung cancer by ROSE. Navigation to peripheral lung lesions was achieved with a combination of fluoroscopy, radial-probe EBUS (UM-S20-20R, Olympus, Tokyo, Japan), a “hybrid” bronchoscope (BF-MP160F, Olympus Ltd., Tokyo, Japan), and electromagnetic navigational bronchoscopy (super Dimension System, Covidien, Mansfield, MA, USA). EBUS-TBNA was performed with EBUS bronchoscope BF-UC-180F (Olympus Ltd., Tokyo, Japan) and a dedicated 22 G needle (NA-201SX; Olympus Ltd, Tokyo, Japan). Systematic nodal staging was defined as EBUS staging in N3-N2-N1 fashion (starting at contralateral hilar LN) in an attempt to rule out both mediastinal and hilar lymphadenopathies, with sampling of any LN measuring at least 5 mm in short axis by ultrasound. LN samples were considered “inadequate” if ROSE only showed blood, bronchial cells, or necrosis, and “adequate” if it showed lymphocytes, granulomas or malignancy. FMs were placed after ROSE results were available confirming diagnosis of malignancy and N0 disease. Two types of FM were utilized: 0.9 mm × 3 mm linear gold markers (CIVCO, Orange City, IA, USA) and 0.8 mm × 3.5 mm gold markers with a 4 mm nitinol anchoring coil (superLock Cobra, Covidien, Mansfield, MA, USA). Markers were deployed either with a microbiology protected specimen brush (Disposable Microbiology Brush, ConMed, Utica, NY, USA), or with a dedicated FM delivery kit (superDimension, Marker Delivery Kit, Covidien Mansfield, MA, USA) under fluoroscopic guidance as previously described (9).
Statistical analysis
Data are reported as mean and standard deviation (SD) for normally distributed continuous variables and as median and interquartile range (IQR) for non-normally distributed continuous variables. Categorical variables are reported as median and mode or number and percent as appropriate. Analysis was performed using Excel 2010 for Windows (Microsoft, Redmond, WA, USA).
Results
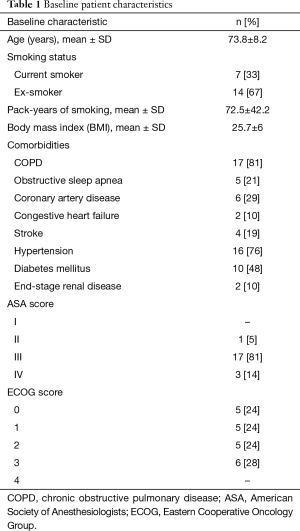
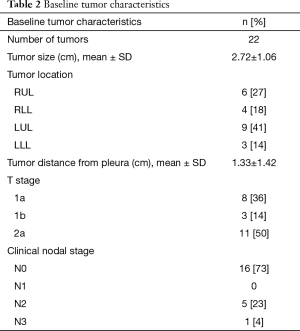
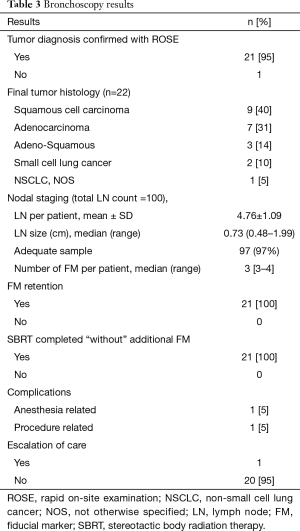
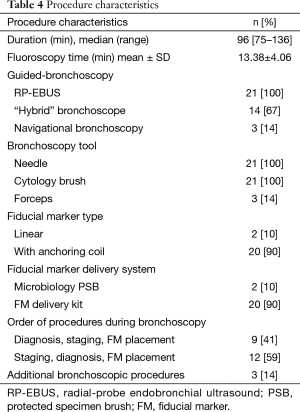
A total of 21 patients met our inclusion criteria, with one patient having 2 tumors (considered as synchronous primaries by a multidisciplinary tumor board). The demographics and baseline medical characteristics are summarized in Table 1. All our patients were male. Ninety five percent of our patients had an ASA score of at least 3. A total of 22 tumors were diagnosed with a size of 2.72±1.06 cm (mean ± SD). Distance from the pleura was 1.33±1.42 cm (mean ± SD). Other tumor characteristics are depicted in Table 2, and histologic diagnosis is described in Table 3. Twenty-one patients underwent bronchoscopy with a median duration of 96 minutes (min), and a range of 75 to 136 min. Three of these 21 patients required additional bronchoscopic procedures due to incidental finding of small endobronchial lesions. Trainees were actively involved in all steps of bronchoscopy in the 21 cases. All procedures were performed in an outpatient setting. Fluoroscopy and RP-EBUS were utilized in 100% of the cases to locate peripheral lung lesions, in combination with “hybrid”/ultrathin scope (14 cases) as well as electromagnetic navigational bronchoscopy (3 cases). Needle and cytology brushes were utilized in all cases (since cytology samples were needed for ROSE), with biopsy forceps only utilized in 2 patients. For other procedure characteristics refer to Table 4. ROSE confirmed diagnosis of malignancy in 21 out of 22 tumors (95%), and it was highly suspicious for it in the remaining one (Table 3). A total of 100 LN were sampled in 21 patients by EBUS-TBNA, with 95% of the patients having at least 4 LN sampled. Sample adequacy rate was 97%, and all LN were negative for malignancy. A total of 71 FM were placed in 22 tumors, with only 2 tumors receiving 4 markers and the rest receiving 3. The majority of the patients (90%) had FM with anchoring Nitinol coil placed via a dedicated FM delivery kit. All patients retained their FM and underwent SBRT without the need of additional markers. One patient developed chills and fever during his initial phase of recovery from anesthesia, which resolved spontaneously. This patient was still admitted for 24-hour observation and accounted for the one case of escalation of care. One patient developed hemoptysis 24 hours post bronchoscopy after resuming his anticoagulation, and it resolved within 3 days. There were no major complications.
Full table
Full table
Full table
Full table
Discussion
Our study shows that a single bronchoscopy obtaining diagnosis of peripheral lung nodules, systematically staging mediastinal and hilar LN, and placing FMs can be safely performed though it results in lengthy procedures. This “all-in-one” approach could potentially expedite management of lung cancer and reduce rate of complications, in particular those associated with CT-guided procedures.
Bronchoscopic diagnosis of peripheral lung nodules has a yield of approximately 70% with most guided-bronchoscopy techniques (RP-EBUS, electromagnetic and non- electromagnetic navigational bronchoscopy), with a much lower rate of pneumothorax than CT-guided biopsies (7). Hence, it has become an attractive alternative when nodules seem to be within bronchoscopic reach. In addition, bronchoscopy can provide nodal staging when indicated, making it the procedure of choice when the above conditions are met (8). Though there have been prior reports of bronchoscopic placement of FMs in combination with mediastinal staging or diagnosis, our study differs from these in several ways. We found a total of 8 studies reporting bronchoscopic placement of FMs for SBRT (Table 5). Four of these 8 studies only focus on the placement of FMs (9,10,13,15), one study reports mediastinal staging and FM placement (12), two studies report diagnosis and FM placement (11,16), and only one study reports diagnosis, staging, and FM placement (14). The study from Sarwate and coworkers reports both on nodal staging and placing of FMs in patients with a previous diagnosis of lung cancer (12). The extent of their nodal staging by EBUS differs substantially from ours. They report sampling 92 LN in 59 patients (average of <2 LN per patient) while we sampled 100 LN in 21 patients (average of almost 5 LN per patient). As bronchoscopists we are aware that it is very common to find LN that are 5 or more mm in short axis. When performing staging in a systematic fashion from N3 to N2 to N1, typically multiple nodes that will meet criteria for biopsy will be found. Our thorough staging is, however, associated with longer procedures. Both studies by Schroeder and Steinfort report on diagnosis and FM placement without staging (11,16). Of note, the former study states that diagnosis was performed on-site in some of the patients (without stating a specific number), and the latter obtained diagnosis in 10 of their 16 patients. In comparison with these two studies we added our extensive nodal staging and we achieved diagnosis in 100% of our cases. Trying to achieve diagnosis may entail additional samples, longer waits for ROSE results, and therefore longer procedures. Lastly, the study by Nabavizedah and coworkers “partially” combined all three procedures: diagnosis, staging and FM placement (14). Of note, diagnosis was obtained in roughly 30% of their patients, and there is no description of their nodal staging (i.e., number of sampled LN). Unlike Nabavizedah and coworkers, we report diagnosis in 100% of our patients and we describe a thorough systematic nodal staging.

Full table
A major finding of our study was the prolonged length of our procedures. We believe this can be easily explained when considering that diagnosis was achieved in all cases and staging was extensively performed. The combination of these two factors leads to multiple cytology samples and longer waits for ROSE results. FMs were not placed until diagnosis was confirmed and all LN samples were deemed negative for malignancy. In addition, trainees were involved in 100% of our cases, and trainee involvement has been proven to lengthen procedures (17). Moreover, three of our patients had additional bronchoscopic procedures. They were all incidentally found to have small endobronchial lesions for which they underwent RP-EBUS to assess the depth of invasion, biopsy with touch-prep and ROSE diagnosis, and treatment with argon plasma coagulation. And one patient had 2 separate tumors that needed diagnosis and FMs. These four cases were found to be the longest ones (all above 100 minutes).
The majority of the above mentioned studies on bronchoscopic placement of FM were done under moderate sedation. However, the study by Nabavizedah and coworkers, which was the only one that partially combined diagnosis, staging, and FM placement -reporting procedure times from 60–90 minutes- was performed under general anesthesia (14). We believe that it would be very difficult to fully perform these three procedures—including an extensive nodal staging—under moderate sedation, particularly when trainee education is involved due to the requirement for longer procedure times.
The retention rate for our FMs was 100%. Though we did not measure the distance from our FMs to the tumors, all of our patients underwent SBRT successfully without the need of additional markers. The absolute lack of FM migration in our study differs from prior publications (10,15,16). We believe that this difference resides in the use of specialized markers with an anchoring nitinol coil, though this is so far an assumption. Slightly more than half of our patients had staging done first, followed by diagnosis and FM placement, and 41% had diagnosis first, followed by staging and FM placement. In those patients whose LN were enlarged, or in whom the likelihood of malignancy (in their lung nodules) was very high, staging was performed first to potentially avoid a second procedure. When the likelihood of malignancy was not as high and an alternative diagnosis was suspected, diagnosis of peripheral lesions was the first procedure.
In addition to COPD, several of our patients had had recent acute coronary syndromes and stroke making them poor candidates for thoracic surgery. Despite our long procedures under general anesthesia in such a high-risk population, our patients tolerated the procedures well, without any major complication. Surprisingly, although all procedures were performed under positive pressure ventilation, there were no pneumothorax. One of our patients experienced hemoptysis in the context of anticoagulation for a recent pulmonary embolism. Though the amount of hemoptysis was small, since it was persistent, an inferior vena cava filter was placed.
The major limitations of our study are its retrospective nature and the small sample size. Also, the patient cohort comes from a single institution with high experience in advanced bronchoscopic techniques, which may impair the generalizability of the results. The involvement of trainees in all cases may impair the generalizability of the results as well. We would also like the highlight that the diagnostic yield of 100% in this population was due to selection bias, since only patients with confirmed diagnosis would subsequently had mediastinal staging and placement of FM during the same bronchoscopic procedure.
To the best of our knowledge, this is the first report in which an entire group of high risk-patients underwent a combination of peripheral bronchoscopy with diagnosis of lung cancer, extensive systematic nodal staging with EBUS-TBNA, and placement of FMs in the same bronchoscopic procedure. Procedures were lengthy but there were no major complications associated with them. With SBRT becoming the standard of care for patients with medically inoperable early stage lung cancer, our strategy of an “all-in-one” bronchoscopy could potentially expedite treatment, decrease complications, and reduce costs. Further prospective studies are needed to corroborate our findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ricardi U, Badellino S, Filippi AR. Stereotactic radiotherapy for early stage non-small cell lung cancer. Radiat Oncol J 2015;33:57-65. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Jung IH, Song SY, Jung J, et al. Clinical outcome of fiducial-less CyberKnife radiosurgery for stage I non-small cell lung cancer. Radiat Oncol J 2015;33:89-97. [Crossref] [PubMed]
- van der Voort van Zyp NC, Prévost JB, Hoogeman MS, Praag J, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol 2009;91:296-300. [Crossref] [PubMed]
- Nuyttens JJ, Prévost JB, Praag J, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol 2006;45:961-5. [Crossref] [PubMed]
- Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol 2007;2:39. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Harley DP, Krimsky WS, Sarkar S, et al. Fiducial marker placement using endobronchial ultrasound and navigational bronchoscopy for stereotactic radiosurgery: an alternative strategy. Ann Thorac Surg 2010;89:368-73; discussion 373-4. [Crossref] [PubMed]
- Schroeder C, Hejal R, Linden PA. Coil spring fiducial markers placed safely using navigation bronchoscopy in inoperable patients allows accurate delivery of CyberKnife stereotactic radiosurgery. J Thorac Cardiovasc Surg 2010;140:1137-42. [Crossref] [PubMed]
- Sarwate D, Sarkar S, Krimsky WS, et al. Optimization of mediastinal staging in potential candidates for stereotactic radiosurgery of the chest. J Thorac Cardiovasc Surg 2012;144:81-6. [Crossref] [PubMed]
- Hagmeyer L, Priegnitz C, Kocher M, et al. Fiducial marker placement via conventional or electromagnetic navigation bronchoscopy (ENB): an interdisciplinary approach to the curative management of lung cancer. Clin Respir J 2016;10:291-7. [Crossref] [PubMed]
- Nabavizadeh N, Zhang J, Elliott DA, et al. Electromagnetic navigational bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy: analysis of safety, feasibility, and interfraction stability. J Bronchology Interv Pulmonol 2014;21:123-30. [Crossref] [PubMed]
- Bolton WD, Richey J, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy: A Safe and Effective Method for Fiducial Marker Placement in Lung Cancer Patients. Am Surg 2015;81:659-62. [PubMed]
- Steinfort DP, Siva S, Kron T, et al. Multimodality guidance for accurate bronchoscopic insertion of fiducial markers. J Thorac Oncol 2015;10:324-30. [Crossref] [PubMed]
- Stather DR, MacEachern P, Chee A, et al. Trainee impact on procedural complications: an analysis of 967 consecutive flexible bronchoscopy procedures in an interventional pulmonology practice. Respiration 2013;85:422-8. [Crossref] [PubMed]


