
Analyzing the impact of minimally invasive surgical approaches on post-operative outcomes of pneumonectomy and sleeve lobectomy patients
Highlight box
Key findings
• Video-assisted thoracoscopic surgical technique does not compromise post-operative outcomes for NSCLC patients.
What is known and what is new?
• Early-stage NSCLC can be treated with surgery including sleeve lobectomy and pneumonectomy. Patient undergoing sleeve lobectomy have improved lost-term survival and fewer complications compared to patients undergoing pneumonectomy. Minimally invasive surgical techniques are gaining popularity due to decreased morbidity and patient preference.
• VATS sleeve lobectomy and pneumonectomy are feasible operations and performing NSCLC surgery using a VATS approach does not compromise survival.
What is the implication, and what should change now?
• Based on this data more surgeons may be influenced to adapt minimally invasive surgical techniques for the treatment of NSCLC.
Introduction
Lung cancer remains one of the deadliest cancers leading to death in 135,720 people in 2020 (1). Early-stage lung cancer is often treated with complete surgical resection (2). Later-stage cancers may also be treated with neoadjuvant therapy, adjuvant chemotherapy, immunotherapy, or radiation (2). Common surgical treatments include wedge resections and anatomic resections such as: segmentectomy, lobectomy, and pneumonectomy (PN). The approach chosen for the treatment of lung cancer depends on the tumor stage and location (2). Other factors that are often considered when deciding surgical management of patients is their functional status, results from pulmonary function tests, and at times patients preference (2).
Sleeve lobectomies (SL) are a type of lobectomy where a section of bronchus is excised and the residual stump is anastomosed with part of the bronchus distally (3). Rates of SL and other lung sparing alternatives are increasing due to the decreased morbidity associated with the procedure (4-7). The merits of choosing SL over PN, when the patient’s disease is conducive, have been analyzed thoroughly. Studies have shown lower mortality rates and preservation of oncologic outcomes (5,8-10). Abdelsattar et al. showed that when analyzing national databases including 23,964 patients, including 1,713 SL patients, there was improved long-term survival and decreased mortality in the SL group (11). The improved outcomes were shown to remain in patients above 70 years old (12,13). The results also hold following induction therapy (14). The conclusion can be made that SL should be chosen over PN when patient characteristics allow.
In addition to increased utilization of parenchymal-sparing operations, surgeons have endorsed minimally invasive approaches such as video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracic surgery (RATS). The advantages of a minimally invasive approach include decreased post-operative morbidity, decreased length of stay, decreased estimated blood volume (EBL), and improved post-operative quality of life (15-19). The analysis of 78 paired cases of SL by a thoracotomy approach vs. a VATS approach showed no significant difference between the groups in overall survival (OS) and recurrence-free survival (RFS) (16). The VATS group was able to boast lower EBL, shorter drain dwell, and shorter hospital stay (16). Nwogu et al. reported early outcomes on VATS PN in 2006 at Roswell Park (20) which followed that of Craig in 1995 (21) and Conlan in 2003 (22). They showed safety, feasibility, and no compromise in oncologic outcomes. These results suggest that this procedure has utility in patients with anatomy that would make a SL suboptimal. This study provides additional support for the adoption of VATS technique for SL and PN operations.
Roviaro performed the first VATS lobectomy in 1992 when he removed the right lower lobe of a patient afflicted with adenocarcinoma (23). Santambrogio performed the first VATS SL in 2002 to treat disease in the left lower lobe bronchus (24). Due to concerns about safety and oncologic outcomes, VATS SL has not been as widely accepted as VATS PN (25). Though much of the current literature is single case reports or small series, the larger reviews have supported the use of VATS to perform SL. In an article reviewing 281 cases of VATS SL, there was no increased rate of complications or mortality (26). There was no compromise to oncologic outcomes observed (26).
We aim to investigate if there is a relationship between surgical approach and post-operative NSCLC patient outcomes. Based on previous works in the field we expect to see PN patients having worse peri- and post-operative outcomes when compared with SL patients. We also do not expect the adoption of a minimally invasive surgical approach to negatively affect these outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-654/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (No. BDR-055715) and individual consent for this retrospective analysis was waived. We reviewed SL and pneumonectomies performed for NSCLC at Roswell Park Comprehensive Cancer Center over a 10-year period from 2007 to 2017. We compared patients who underwent a PN compared to those who had a SL. Patients were included in the study if they underwent a SL or PN at Roswell Park Comprehensive Cancer Center between 2007–2017 due to oncologic reasons. Patients were excluded from analysis if they underwent a SL or PN for other reasons such as infection. The open PN and SL were performed using standard surgical procedure. Of note, when performing VATS PN we commonly adopt procedures described by Demmy et al. (27). Patient follow up was determined by arrival to scheduled follow up appointments at the Roswell Park Thoracic Surgery Clinic. Patients were considered lost to follow up if they failed to arrive to scheduled appointments. Outcome variables analyzed include: 30-day and 90-day mortality, complication rate, local recurrence rate, and survival. Due to the retrospective nature of this study, we cannot ensure that there was no bias in the reporting of complications. Due to this, we aimed to analyze variables that had quantitative reporting rather than qualitative. We hope that by doing this we helped to alleviate observer bias and have the ability to carry out straight forward analyses looking for differences between the SL and PN groups. The 30-day and 90-day mortality were calculated by analyzing the number of patient deaths that occurred within 30 and 90 days of the operation. To analyze complications, we enumerated all complications [return to operating room, acute respiratory distress syndrome (ARDS), bronchopleural fistula (BPF), pneumonia, vocal cord paralysis, empyema, chylothorax, atrial fibrillation, reintubation]. If patients had multiple complications each individual complication was recorded. When analyzing local recurrence, we classified it as disease within the ipsilateral chest cavity. OS was counted from the time of surgery until a censoring event that was defined as death, secondary to any cause, or their last follow-up visit. If patients were lost to follow up, they were counted as deceased following their last follow up appointment. We converted our survival in days to survival in months by dividing the answer in days by 30.437 (average number of days in a month). The data set was ended in 2017 to allow at least three-year survival data on patients.
Statistical analysis
Differences in mortality were analyzed using the Kaplan-Meier method using the log rank test to compare the PN and SL groups. A two-tailed Z test for difference in proportions was done to analyze complications, local recurrence, 30-day and 90-day mortality. To analyze the impact of a minimally invasive approach, age at time of surgery, patient sex, tumor stage, and tumor histology on survival we performed multivariate analysis.
Results
A total of 108 patients underwent SL or PN for NSCLC during this ten-year period. Demographic information can be found in Table 1.
Table 1
| Variables | Total PN (n=74) | Total SL (n=34) | Open PN (n=18) | VATS PN (n=56) | Open SL (n=29) | VATS SL (n=5) |
|---|---|---|---|---|---|---|
| Pathologic stage, n (%) | ||||||
| I | 16 (21.6) | 9 (26.5) | 3 (16.7) | 13 (23.2) | 9 (31.0) | 0 |
| II | 31 (41.9) | 15 (44.1) | 9 (50.0) | 22 (39.3) | 11 (37.9) | 4 (80.0) |
| III | 27 (36.5) | 10 (29.4) | 6 (33.3) | 21 (37.5) | 9 (31.0) | 1 (20.0) |
| Histology, n (%) | ||||||
| Squamous | 40 (54.1) | 23 (67.6) | 11 (61.1) | 29 (51.8) | 21 (72.4) | 2 (40.0) |
| Adeno | 32 (43.2) | 10 (29.4) | 6 (33.3) | 26 (46.4) | 8 (27.6) | 2 (40.0) |
| Large cell | 2 (2.7) | 1 (2.9) | 1 (5.6) | 1 (1.8) | 0 | 1 (20.0) |
| Average age at time of surgery | 62.9 | 62 | 59.4 | 64.0 | 62.1 | 61.2 |
| Patient sex, n (%) | ||||||
| Male | 42 (56.8) | 21 (61.8) | 13 (72.2) | 29 (51.8) | 16 (55.2) | 5 (100.0) |
| Female | 32 (43.2) | 13 (38.2) | 5 (27.8) | 27 (48.2) | 13 (44.8) | 0 |
PN, pneumonectomy; SL, sleeve lobectomy; VATS, video-assisted thoracoscopic surgery; Adeno, adenocarcinoma; Squamous, squamous cell carcinoma.
There was a statistically significant difference in 90-day mortality between the SL and PN groups (P=0.007). There was no difference in 30-day mortality between the SL and PN groups (P=0.064) (Table 2).
Table 2
| Death | Total PN (n=74) | VATS PN (n=56) | Open PN (n=18) | Total SL (n=34) | Open SL (n=29) | VATS SL (n=5) |
|---|---|---|---|---|---|---|
| <30-day | 7 (9.5) | 6 (10.7) | 1 (5.6) | 0 | 0 | 0 |
| 31- to 90-day | 7 (9.5) | 2 (3.6) | 5 (27.8) | 0 | 0 | 0 |
Data are expressed as N (%). PN, pneumonectomy; SL, sleeve lobectomy; VATS, video-assisted thoracoscopic surgery.
We then analyzed complication rates between cohorts. When looking at the PN patients there were 47 complications and when looking at the SL group there were 17 complications (P=0.234). The complications and their respective rates can be seen in Table 3.
Table 3
| Complication | Total PN (n=74) | Open PN (n=18) | VATS PN (n=56) | Total SL (n=34) | Open SL (n=29) | VATS SL (n=5) |
|---|---|---|---|---|---|---|
| Return to OR | 10 (13.5) | 2 (11.1) | 8 (14.3) | 1 (2.9) | 1 (3.4) | 0 |
| ARDS | 2 (2.7) | 2 (11.1) | 0 | 0 | 0 | 0 |
| BPF | 5 (6.8) | 1 (5.6) | 4 (7.1) | 0 | 0 | 0 |
| Pneumonia | 8 (10.8) | 2 (11.1) | 6 (10.7) | 6 (17.6) | 5 (17.2) | 1 (20.0) |
| Vocal cord paralysis | 8 (10.8) | 3 (16.7) | 5 (8.9) | 5 (14.7) | 5 (17.2) | 0 |
| Empyema | 3 (4.1) | 0 | 3 (5.4) | 0 | 0 | 0 |
| Chylothorax | 1 (1.4) | 1 (5.6) | 0 | 0 | 0 | 0 |
| Afib | 8 (10.8) | 1 (5.6) | 7 (12.5) | 3 (8.8) | 3 (10.3) | 0 |
| Reintubation | 2 (2.7) | 2 (11.1) | 0 | 2 (5.9) | 1 (3.4) | 1 (20.0) |
| Total | 47 | 14 | 33 | 17 | 15 | 2 |
Data are expressed as N (%) or N. PN, pneumonectomy; VATS, video-assisted thoracoscopic surgery; SL, sleeve lobectomy; OR, operating room; ARDS, acute respiratory distress syndrome; BPF, bronchopleural fistula; Afib, atrial fibrillation.
We then analyzed the differences in local recurrence rates. There was no significant difference between local recurrence rates (P=0.779) and distant recurrence rates (P=0.576) between the PN and SL groups (Table 4).
Table 4
| Metastasis | Total PN (n=74) | Open PN (n=18) | VATS PN (n=56) | Total SL (n=34) | Open SL (n=29) | VATS SL (n=5) |
|---|---|---|---|---|---|---|
| Local* | 3 (4.1) | 0 | 3 (5.4) | 1 (2.9) | 1 (3.4) | 0 |
| Distant# | 18 (24.3) | 6 (33.3) | 12 (21.4) | 10 (29.4) | 8 (27.6) | 2 (40.0) |
*, open vs. VATS: P=0.281. #, open vs. VATS: P=0.576. Data are expressed as N (%). PN, pneumonectomy; VATS, video-assisted thoracoscopic surgery; SL, sleeve lobectomy.
The PN group had a median disease-specific survival of 23.6 months (95% CI: 3.8–43.4 months). The SL group had a median disease-specific survival of 60.7 months (95% CI: 43.4–78.2 months) (Figure 1).
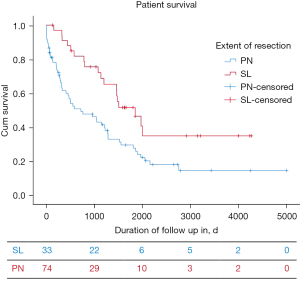
To determine the impact of the extent of surgical resection (SL vs. PN), surgical approach (open vs. VATS), patient sex, age at the time of surgery, tumor histology (adenocarcinoma, squamous, other), and tumor stage on median survival we performed a multivariable analysis. We found that SL patients had superior survival compared to PN (P<0.001) and tumor stage (P=0.036) were significantly associated with survival with earlier-stage patients having better survival. We were also able to conclude that using a minimally invasive surgical approach did not compromise long-term survival compared to open surgical approaches (P=0.053) (Table 5).
Table 5
| Variable | SE | df | sig | Odds ratio (95% CI) |
|---|---|---|---|---|
| Extent of resection (PN vs. SL) | 0.323 | 1 | 0.000 | 3.09 (1.62–5.89) |
| Open vs. VATS | 0.281 | 1 | 0.053 | 1.87 (1.06–3.27) |
| Sex | 0.012 | 1 | 0.425 | 0.98 (0.96–1.00) |
| Age (at time of surgery) | 0.011 | 1 | 0.069 | 1.02 (1.00–1.04) |
| Histology | 2 | 0.295 | ||
| Adeno vs. others | 0.259 | 1 | 0.443 | 0.82 (0.49–1.38) |
| Squam vs. others | 1.019 | 1 | 0.157 | 0.24 (0.03–1.81) |
| Stage | 0.176 | 1 | 0.036 | 1.51 (1.06–2.14) |
Statistical significance: P<0.05. df, degrees of freedom; SE, standard error; sig, significance; PN, pneumonectomy; SL, sleeve lobectomy; VATS, video-assisted thoracoscopic surgery; Adeno, adenocarcinoma; Squam, squamous cell carcinoma.
Discussion
When comparing our PN outcomes in current literature we had similar results. In a study of 1,160 patients, Skrzypczak et al. experienced 30-day mortality of 4% and a five-year survival of 45% (28). They also experienced 56.7% morbidity, as defined by the patients experiencing at least one complication (28). Gu et al. analyzed 406 patients and experiences a 30-day mortality of 3.2% and saw a five-year survival of 32.5% (29). They observed a 36.7% morbidity (29). Ludwig et al. studied 194 PN patients and observed a 30-day mortality of 4.6%, a 5-year survival rate of 27% and their patients had a morbidity rate of 23% (9). Moore et al. reported a 30-day mortality of 5.1% in 15,524 patients and a 90-day mortality of 5.1% in a study of 15,455 patients (30).
When analyzing SL outcomes to current literature we had similar results. Chen et al. observed 30-day mortality of .6% and 90-day mortality of 0.9% in a patient cohort of 964 (31). They also reported a morbidity of 4.36% and a five-year survival of 62.7% (31). Yazgan et al. reported a five-year survival of 50.9%, a 30-day mortality of 1.2% and a 90-day mortality of 2.4% for a group of 80 patients (32). In patients undergoing open SL, Mayne et al. reported a 5-year survival of 79%, a 30-day mortality of 4.5%, and a 90-day mortality of 6.8% (33).
Conclusions
When considering NSCLC patients, performing a SL rather than a PN was related to improved median survival and 90-day mortality. And does not negatively impact 30-day mortality, complications rate, or local recurrence as seen by a lack of statistical differences in these variables when the patients who underwent SL were compared to those who underwent PN. Median survival was also increased when the patient had a lower tumor stage.
Finally, survival was not compromised when a patient had their surgery performed using a minimally invasive approach. We are able to conclude that the addition of the minimally invasive cohort did not compromise post-operative outcomes in patients undergoing SL or PN to treat NSCLC.
One of the shortfalls of our review is that we had a relatively few numbers of patients in the VATS SL group. This therefore may have minimized the impact of this group either positively or negatively on the total group of SL. This study was done at a single institution therefore further work needs to be completed to ensure external validity.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-654/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-654/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-654/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-654/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Collins LG, Haines C, Perkel R, et al. Lung cancer: diagnosis and management. Am Fam Physician 2007;75:56-63. [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-81. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Ma QL, Guo YQ, Shi B, et al. For non-small cell lung cancer with T3 (central) disease, sleeve lobectomy or pneumonectomy? J Thorac Dis 2016;8:1227-33. [Crossref] [PubMed]
- Andersson SE, Rauma VH, Sihvo EI, et al. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: a propensity-matched analysis of long-term results, survival and quality of life. J Thorac Dis 2015;7:1742-8. [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Stallard J, Loberg A, Dunning J, et al. Is a sleeve lobectomy significantly better than a pneumonectomy? Interact Cardiovasc Thorac Surg 2010;11:660-6. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Pan X, Tantai J, Lin L, et al. Comparison of short and long-term results between sleeve resection and pneumonectomy in lung cancer patients over 70 years old: 10 years experience from a single institution in China. Thorac Cancer 2014;5:494-9. [Crossref] [PubMed]
- Melloul E, Egger B, Krueger T, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg 2008;7:986-9. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Gao HJ, Jiang ZH, Gong L, et al. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann Thorac Surg 2019;108:1072-9. [Crossref] [PubMed]
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Craig SR, Walker WS. Initial experience of video assisted thoracoscopic pneumonectomy. Thorax 1995;50:392-5. [Crossref] [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Davoli F, Bertolaccini L, Pardolesi A, et al. Video-assisted thoracoscopic surgery bronchial sleeve lobectomy. J Vis Surg 2017;3:41. [Crossref] [PubMed]
- Zhong Y, Wang Y, Hu X, et al. A systematic review and meta-analysis of thoracoscopic versus thoracotomy sleeve lobectomy. J Thorac Dis 2020;12:5678-90. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Technique of video-assisted thoracoscopic left pneumonectomy. J Vis Surg 2017;3:32. [Crossref] [PubMed]
- Skrzypczak PJ, Roszak M, Kasprzyk M, et al. Pneumonectomy - permanent injury or still effective method of treatment? Early and long-term results and quality of life after pneumonectomy due to non-small cell lung cancer. Kardiochir Torakochirurgia Pol 2019;16:7-12. [Crossref] [PubMed]
- Gu C, Wang R, Pan X, et al. Comprehensive study of prognostic risk factors of patients underwent pneumonectomy. J Cancer 2017;8:2097-103. [Crossref] [PubMed]
- Moore CB, Cox ML, Mulvihill MS, et al. Challenging 30-day mortality as a site-specific quality metric in non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:570-578.e3. [Crossref] [PubMed]
- Chen J, Soultanis KM, Sun F, et al. Outcomes of sleeve lobectomy versus pneumonectomy: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;162:1619-1628.e4. [Crossref] [PubMed]
- Yazgan S, Gürsoy S, Üçvet A, et al. Long-term results of sleeve lobectomy with continuous suture technique in non-small cell lung cancer. Turk Gogus Kalp Damar Cerrahisi Derg 2019;27:93-100. [Crossref] [PubMed]
- Mayne NR, Darling AJ, Raman V, et al. Perioperative Outcomes and 5-year Survival After Open versus Thoracoscopic Sleeve Resection for Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:522-30. [Crossref] [PubMed]


