
Sepsis-related immunosuppression: a bibliometric analysis
Highlight box
Key findings
• More and more studies are being published on sepsis-related immunosuppression, but the research is largely being conducted in developed countries in Europe and America. Chinese researchers need to carry out more collaborative research.
What is known and what is new?
• Sepsis is one of the main causes of death in critically ill patients. Immune dysfunction is key to the occurrence and development of sepsis.
• The number of articles on sepsis-related immunosuppression increased annually. Moldawer and Chaudry from the United States (US) had engaged in the most collaborations with other researchers. The journals that publish literature in this field are mainly journals related to critical care medicine, and the core journals included Shock, Critical Care, and Critical Care Medicine.
What is the implication, and what should change now?
• Chinese researchers need to carry out more collaborative research.
Introduction
Sepsis is an organ dysfunction caused by the uncontrolled response of the body to infection. It is one of the main causes of death in critically ill patients and seriously affects the prognosis of critically ill patients (1). In 2017, 48.9 million new sepsis cases and 11 million deaths, accounting for 19.7% of global deaths, were reported worldwide (2). Since the launch of the “Save Sepsis Campaign” in 2002, some progress has been made in both the basic and clinical research on sepsis, but the morbidity and mortality rates of sepsis have not decreased significantly (3), which may be related to immunosuppression in patients with sepsis.
Immune dysfunction is key to the occurrence and development of sepsis and is mainly manifested as an imbalance between pro-inflammatory and anti-inflammatory responses (4,5). The pro-inflammatory response can cause a storm of inflammatory factors, while the anti-inflammatory response can lead to immunosuppression. Immunosuppression is an important factor leading to the occurrence of secondary infections in the body and is also the key to poor prognosis in patients with sepsis (6,7). Some studies have highlighted the important role of the NLRP3 inflammasome in sepsis. This inflammasome regulates the activation of caspase-1 and the production and secretion of potent pro-inflammatory cytokines (8,9). The occurrence and development of immunosuppression in sepsis are mainly manifested by changes in anti-inflammatory cytokines, immune cells, and immune co-stimulatory inhibitory molecules (10-12). At present, the main clinical drugs used in this field include thymosin α1, interleukin (IL)-7, oxidized phospholipids, necrosulfanilamide and high mobility group box-1 (HMGB1) antibodies, anti-death pathway-related antibodies, and anti-programmed cell death receptor 1 (PD-1), and programmed cell death ligand 1 (PD-L1) antibodies (13-16). This study sought to conduct a preliminarily analysis of the current research status in this field through bibliometric research.
Methods
Literature search
Similar to other bibliometric studies (17,18), we searched the Science Citation Index Expanded (SCI-E) database in the Web of Science Core Collection, and the time was set from the inception of the database to the last retrieval time for this study (i.e., May 21, 2022). In this study, the topic search was used, and the search terms were sepsis and immunosuppression. We searched for “sepsis”, and we then searched for “immunosuppression” in the previous results to obtain the final search results.
Data analysis
On the search page of SCI-E, we selected document types, topic directions, MeSH subject headings, MeSH qualifiers, keywords, authors, journals, countries, research institutions, languages, etc. to obtain the distribution results. All duplicate records were manually removed. We analyzed the use of keywords in the literature and the centrality of authors, countries, and research institutions.
Statistical analysis
This study mainly describes the current status of research on sepsis and immunosuppression, and the data are expressed in numbers and percentages (n, %).
Results
General information
A total of 4,132 articles were identified in the search of the database over the search period of 1900 to May 21, 2022. These articles cited a total of 81,267 references, including 2,023 self-citations. At the time of our analysis, these articles have been cited 114,138 times, with an average of 27.62 citations per article and an H-index of 139. The types of documents included articles, reviews, case reports, meeting papers, abstracts, clinical trials, and editorials (Figure 1), of which articles represented the largest number. However, in some instances, the same article had multiple classifications. In addition to clinical trials being classified as original articles, some systematic reviews, conference papers, and case reports were also classified as original articles. In terms of topic classification, any given article may have also been classified as being associated with multiple disciplines; however, the most common topics were immunology and infectious disease (Figure 2). According to the annual analysis, there was an obvious trend whereby the number of articles published increased year by year (Figure 3). A trend of rapid growth was also observed in the number of citations (Figure 3).
Topic words and qualifiers
The analysis of the MeSH subject headings recorded in these documents revealed that the most used topic heading was “Human”, followed by “Male”, and “Female” (Figure 4). The results of the MeSH qualifier analysis showed that the most used MeSH qualifier was “Immunology”, followed by “Therapeutic use”, and “Metabolism” (Figure 5).
Authors
In the research field of sepsis-related immunosuppression, the author with the most published papers was Monneret from Lyon, France, who specializes in immunology and the pathophysiology of injury-induced immunosuppression, followed by Venet, a colleague of Monneret, and Moldawer from the University of Florida College of Medicine in the United States (US), who specializes in surgery (Figure 6). A further analysis showed that the main specialized areas of the researchers were surgery, followed by anesthesia, and immunology, which were also related to the disciplinary settings of different countries and research institutions (Table 1). The results of the centrality analysis showed that an author’s centrality had a certain relationship with the number of published documents, but it was not the author with the largest number who had the highest centrality score. In this field, Moldawer from the University of Florida College of Medicine, US, had the highest centrality score, followed by Chaudry from the University of Alabama, Birmingham, US.
Table 1
| Rank | Authors | Specialty | Centrality |
|---|---|---|---|
| 1 | Moldawer, Lyle L | Surgery | 0.46 |
| 2 | Chaudry, Irshad H | Surgery | 0.39 |
| 3 | Monneret, Guillaume | Immunology | 0.37 |
| 4 | Venet, Fabienne | Immunology | 0.33 |
| 5 | Moore, Frederick A | Surgery | 0.27 |
| 6 | Efron, Philip A | Surgery | 0.23 |
| 7 | Hotchkiss, RS | Anesthesiology | 0.20 |
| 8 | Ayala, Alfred | Surgery | 0.16 |
| 9 | Rimmele, Thomas | Anesthesiology | 0.11 |
| 10 | Mohr, Alicia M | Surgery | 0.10 |
Journals
The results of the analysis of the journals (Figure 7) revealed that most of the articles in this field had been published in critical care-related journals, such as Shock, Critical Care, and Critical Care Medicine. According to Bradford’s law, the core journals in the field are Shock, Critical Care, Critical Care Medicine, Transplantation Proceedings, and Blood and Frontiers in Immunology.
Countries, institutions, and languages
In this research field, the top 3 countries with the most published papers were the US, China and Germany (Figure 8). However, in the centrality ranking of countries, the top 3 countries were the US, the United Kingdom (UK), and Germany; China was only ranked 7th (Table 2). The institutions with the most published papers were the League of European Research Universities (LERU) and Udice French Research Universities (UFRU). The 3rd and 10th institutions were France and the US, respectively, while Chinese research institutions did not enter the top 10 (Figure 9). The results of the centrality ranking of institutions (Table 3) showed that LERU, INSERM, and UFRU held the 1st to 3rd places, respectively, but there was still no institution in China whose centrality score ranked in the top 10. In terms of the language of publication, in the SCI database, research in this field was dominated by English-language articles (92.9%), followed by Chinese-language articles (2.59%) (Table 4), and a small amount of German-, French-, and Spanish-language articles.
Table 2
| Rank | Countries | Centrality |
|---|---|---|
| 1 | US | 0.26 |
| 2 | UK | 0.20 |
| 3 | Germany | 0.18 |
| 4 | France | 0.15 |
| 5 | Italy | 0.14 |
| 6 | Japan | 0.11 |
| 7 | China | 0.10 |
| 8 | Spain | 0.09 |
| 9 | Canada | 0.08 |
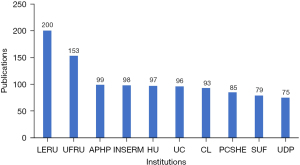
Table 3
| Rank | Institutions | Centrality |
|---|---|---|
| 1 | LERU | 0.34 |
| 2 | INSERM | 0.29 |
| 3 | UFRU | 0.25 |
| 4 | HU | 0.22 |
| 5 | APHP | 0.19 |
| 6 | UC | 0.16 |
| 7 | SUF | 0.12 |
| 8 | PCSHE | 0.11 |
| 9 | UDP | 0.10 |
LERU, League of European Research Universities; UFRU, Udice French Research Universities; APHP, Assistance Publique Hopitaux Paris; INSERM, Institut National De La Sante Et De La Recherche MedicalE; HU, Harvard University; UC, University of California System; CL, Chu Lyon; PCSHE, Pennsylvania Commonwealth System of Higher Education; SUF, State University System of Florida; UDP, Universite De Paris.
Table 4
| Language | Publications | % of 4,132 |
|---|---|---|
| English | 3,805 | 92.09 |
| Chinese | 107 | 2.59 |
| German | 97 | 2.35 |
| French | 55 | 1.33 |
| Spanish | 55 | 1.33 |
| Japanese | 15 | 0.36 |
| Portuguese | 14 | 0.34 |
| Russian | 14 | 0.34 |
| Italian | 12 | 0.29 |
| Polish | 9 | 0.22 |
Keywords
The use of keywords was analyzed, and the results showed (Figure 10) that sepsis and immunosuppression were the most commonly used keywords, while male, female, and elderly were also frequently used keywords. Additionally, infection, septic shock, and inflammation were also commonly used keywords. The results of the centrality analysis (Table 5) showed that the top 3 key words with the highest centrality scores were sepsis, immunosuppression, and infection.
Table 5
| Rank | Keywords | Centrality |
|---|---|---|
| 1 | Sepsis | 0.41 |
| 2 | Immunosuppression | 0.33 |
| 3 | Infection | 0.26 |
| 4 | Inflammation | 0.23 |
| 5 | Male | 0.17 |
| 6 | Female | 0.15 |
| 7 | Septic shock | 0.14 |
| 8 | Immunotherapy | 0.12 |
| 9 | Mechanism | 0.11 |
| 10 | Elderly | 0.10 |
Discussion
In this study, we searched for articles published from 1900 to May 21, 2022 and retrieved a total of 4,132 articles. These articles largely reflect the research profile of sepsis-related immunosuppression. Our findings showed that the research literature in this field is dominated by original articles, followed by some reviews and case reports. The number of documents published has increased year by year, especially after 2013. There were some differences between the results of the subject word analysis and the keyword analysis. The most used subject words were humans, male, and female, while the most used keywords were sepsis, immunosuppression, and male, and the most used MeSH qualifiers were immunology, therapeutic use, and metabolism. An analysis of the authors showed that the most published researcher was Monneret from Lyon, France. The authors of the articles largely specialized in immunology and surgery. In terms of research cooperation, Moldawer and Chaudry from the US had engaged in the most cooperation with other researchers. In relation to the analysis of journals, the results showed that the journals publishing articles in this field were mainly journals related to critical care medicine, and the core journals included Shock, Critical Care, and Critical Care Medicine. In terms of the distribution of countries, most of the researchers came from the US, China, and Germany, but the countries with the most cooperation were the US, the UK, and Germany. In terms of research institutions, the institutions were largely based in Europe and the US. The articles in this field were mainly published in the English language.
At present, research in the field of sepsis-related immunosuppression has generated abundant results and made significant progress (19-21). It is currently thought that sepsis-related immunosuppression mainly involves the innate and adaptive immune systems and is manifested by an increased release of anti-inflammatory cytokines, the apoptosis of immune cells, the decreased expression of human leukocyte antigen DR (HLA-DR), pyroptosis, and the increased expression of PD-1 and PD-L1 (4,22,23).
Anti-inflammatory cytokines mainly include IL-4, IL-10, and IL-37. IL-4 is produced and secreted by activated T cells and mast cells, induces cluster of differentiation (CD)4+ T cells to differentiate into helper T cells 2 (Th2 cells), and promotes self-secretion through positive feedback, while stimulating the release of other anti-inflammatory cytokines, and exerts the biological effect of inhibiting the release of pro-inflammatory cytokines (24). Furthermore, sepsis-related immunosuppression has been proved to be involved in the occurrence of sepsis-associated encephalopathy through multiple pro-inflammatory cytokines (25). Also, this kind of immunosuppression is associated with the neuron dysfunction and final cell death (26).
IL-10 is mainly secreted by mononuclear macrophages and Th2 cells, which can inhibit T cell proliferation and immune effector function, inhibit the release of pro-inflammatory cytokines, and promote the proliferation of immune-suppression cells, including regulatory T cells and bone marrow-derived suppressor cells (27,28). A previous study has shown that IL-10 inhibits the proliferation of T cells in patients with sepsis, reduces the secretion of effector cytokines, and at the same time, promotes the proliferation of regulatory T cells and has an immunosuppressive effect (29).
IL-37, which is a unique member of the IL-1 cytokine family, is also an inhibitor of immune responses and inflammation. It is produced by immune and non-immune cells and inhibits pro-inflammatory cytokine release and antigen presentation (30). Another study found that the expression of IL-37 in patients with sepsis is significantly increased, which can inhibit the proliferation and release of pro-inflammatory cytokines and is closely related to the severity of the inflammatory response (31). Another study showed that IL-37 significantly downregulates the expression of major histocompatibility complex class II molecules and CD86 in septic mice, and inhibits antigen presentation, indicating that IL-37 has immunosuppressive effects in sepsis (32).
Apoptosis refers to the autonomous and orderly death of cells controlled by genes to maintain the stability of the internal environment. There are 2 main types of apoptosis pathways in sepsis; that is, the exogenous and endogenous pathways. In the exogenous pathway, caspase-8 is first activated through the Fas/Fas ligand pathway, and caspase-3 is then activated to exert the biological effect of apoptosis (33). In the endogenous pathway, cytochrome C and caspase activator 1 first form a multimer. Caspase-9 is activated through combination with the multimer to form apoptotic bodies, and finally activates caspase-3, thus generating biological effects in apoptosis. The endogenous pathway, regulated by members of the B-cell lymphoma/leukemia-2 (Bcl-2) family, accelerates apoptosis through the pro-apoptotic protein Bim, while inhibiting apoptosis through anti-apoptotic proteins, such as Bcl-2 (34,35). A previous study found that the expression levels of cytochrome C, Bim, caspase-3, caspase-8, caspase-9, etc. in the sepsis mouse model were significantly increased, while the expression level of Bcl-2 was significantly decreased, which promoted T cell apoptosis (36).
HLA-DR is a major histocompatibility complex class II molecule, which is mainly expressed in monocytes natural killer cells, macrophages, and other innate immune cells, and acquired immune cells, including B lymphocytes and activated T lymphocytes (37). A study by Zhuang et al. has shown that HLA-DR is a good indicator for evaluating the immune status of patients with sepsis and is closely related to a poor clinical prognosis (38). It is currently believed that an HLA-DR level below 30% indicates the existence of immunosuppression (39). Winkler et al. confirmed that the HLA-DR level of patients diagnosed with sepsis in the intensive care unit was 70% lower than that of uninfected preoperative patients, and the HLA-DR level was negatively correlated with the sequential organ failure scores of patients (39). Zhuang et al. found that HLA-DR levels in sepsis patients were negatively correlated with a poor clinical prognosis and immunosuppression (38). Zhou et al. demonstrated that HLA-DR levels were significantly reduced in mice undergoing sepsis compared to sham-operated mice (40).
Pyroptosis is a type of programmed cell death characterized by loss of plasma membrane integrity, cell swelling and rupture, followed by the efflux of intracellular substances and the activation of an inflammatory response. The pyroptosis of immune cells in sepsis mainly exerts biological effects through classical and non-canonical pathways (41). Among them, the classical pathway activates caspase-1 through the inflammasome. Conversely, activated caspase-1 promotes the expression of inflammatory factors (e.g., IL-1β, and IL-18) and HMGB1, recruits inflammatory cells to aggregate, and expands the inflammatory response. However, it cleaves to and activates gasdermin D, which translocates to the cell membrane to form holes, resulting in cell pyroptosis. The non-canonical pathway binds to caspase-4, caspase-5, and caspase-11 through bacterial lipopolysaccharide, activates caspase-1 and gasdermin D, and leads to pyroptosis (42).
A previous study showed that the expression level of caspase-1, the percentage of apoptosis in the peripheral blood mononuclear cells induced by caspase-1, and the level of IL-18 were significantly higher in patients with post-traumatic sepsis than healthy subjects, and the percentage of monocyte apoptosis predicted the occurrence of post-traumatic sepsis (43). PD-1 is a type I transmembrane glycoprotein that is mainly expressed in activated T cells and B cells. The combination of PD-1 on the surface of T cells and PD-L1 on the surface of antigen-presenting cells can lead to T cell exhaustion, which is mainly manifested as weakened effector T cell function, decreased cytokine secretion, and inhibited cell proliferation. Increases in the expression of PD-1 suggest that the clinical prognosis of patients with sepsis is poor (15). The increased expression of PD-1 on the surface of T cells in patients with sepsis has been shown to inhibit the secretion of cytokines, such as tumor necrosis factor (TNF)-α, while the administration of a PD-1 antibody has been shown to promote the release of TNF-α (15). Shao et al. found that PD-1 expression in T cells of patients with septic shock is associated with a poor clinical prognosis and was an independent risk factor for 28-day all-cause mortality (44). Patera et al. found that the expression levels of PD-1 and PD-L1 in neutrophils and monocytes in patients with septic shock were significantly higher than those in patients without infection in the intensive care unit, and PD-1, PD-L1 expression levels were positively correlated with the severity of sepsis and mortality (45).
At present, the treatment of sepsis is mainly based on standard protocols, such as early fluid resuscitation, antibiotic use, and organ support according to the Sepsis-3 guidelines (1). However, strict adherence to standard regimens has not significantly reduced the mortality rate of patients with sepsis (1). Based on clinical understandings of the importance of sepsis-induced immunosuppression in organ damage and death and knowledge of the effects of anti-inflammatory cytokines, immune cell apoptosis, and negative co-stimulatory molecules in patients with sepsis, the use of immunotherapy in the treatment of sepsis has gradually received attention. Combined immunotherapy has achieved initial results in sepsis patients, and has shown great potential and development space. It is expected to become a new treatment strategy in the future and bring a breakthrough in the treatment of sepsis (46).
According to the results of this study, the current research is mainly published in English and mainly occurs in developed countries in Europe and the US, and the participants are mainly immunology-related researchers. We recommend that more clinicians specializing in critical care medicine participate in research in this field, conduct in-depth testing, observe sepsis patients in clinical practice, and carefully evaluate the immune system and functional status of patients. At the same time, various researchers, research institutions, and even countries should cooperate fully and work together to solve the problem of sepsis-related immunosuppression. According to our findings, we believe that the one research hotspot of sepsis in the future might be the immunotherapy in septic patients. As for cooperation, we recommend that Chinese investigators adopt more multicenter clinical research, or conduct more related research with investigators from other different disciplines.
This study had some limitations. First, this study was a bibliometric study, which mainly analyzed the research status of sepsis-related immunosuppression from macroscopic statistical indicators, and an in-depth analysis of each article was not conducted. Second, as each literature may have obvious inconsistencies in the classification of topic headings and the use of keywords, the results of this study may be biased and not reflect the actual situation. Third, this study used the SCI database. This database covers most of the current major medical research journals; however, there are still some journals in other languages that were not included in this study, and thus some important journals may have been missed. We suggest that the SCI database include journals in languages other than English as much as possible to provide more adequate data sources for bibliometric research.
Conclusions
There are many literatures published on sepsis-related immunosuppression, but the research is relatively center in developed countries in Europe and America. There is lack of cooperation among Chinese researchers in this field.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-300/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-300/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 2016;126:23-31. [Crossref] [PubMed]
- Yao YM, Osuchowski MF, Wang JH, et al. Editorial: Immune Dysfunction: An Update of New Immune Cell Subsets and Cytokines in Sepsis. Front Immunol 2021;12:822068. [Crossref] [PubMed]
- Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306:2594-605. [Crossref] [PubMed]
- Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 2012;72:1491-501. [Crossref] [PubMed]
- Gong T, Yang Y, Jin T, et al. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol 2018;39:393-406. [Crossref] [PubMed]
- Katsnelson MA, Lozada-Soto KM, Russo HM, et al. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. Am J Physiol Cell Physiol 2016;311:C83-C100. [Crossref] [PubMed]
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862-74. [Crossref] [PubMed]
- Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 2018;14:121-37. [Crossref] [PubMed]
- Zhu D, Zhu K, Guo S. Identification of key genes related to immune cells in patients with gram-negative sepsis based on weighted gene co-expression network analysis. Ann Transl Med 2022;10:787. [Crossref] [PubMed]
- Morrow KN, Coopersmith CM, Ford ML. IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Front Immunol 2019;10:1982. [Crossref] [PubMed]
- Watanabe H, Son M. The Immune Tolerance Role of the HMGB1-RAGE Axis. Cells 2021;10:564. [Crossref] [PubMed]
- Nakamori Y, Park EJ, Shimaoka M. Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front Immunol 2020;11:624279. [Crossref] [PubMed]
- McBride MA, Patil TK, Bohannon JK, et al. Immune Checkpoints: Novel Therapeutic Targets to Attenuate Sepsis-Induced Immunosuppression. Front Immunol 2020;11:624272. [Crossref] [PubMed]
- Antequera A, Madrid-Pascual O, Solà I, et al. Female under-representation in sepsis studies: a bibliometric analysis of systematic reviews and guidelines. J Clin Epidemiol 2020;126:26-36. [Crossref] [PubMed]
- Evangelatos N, Satyamoorthy K, Levidou G, et al. Multi-Omics Research Trends in Sepsis: A Bibliometric, Comparative Analysis Between the United States, the European Union 28 Member States, and China. OMICS 2018;22:190-7. [Crossref] [PubMed]
- Córneo EDS, Michels M, Dal-Pizzol F. Sepsis, immunosuppression and the role of epigenetic mechanisms. Expert Rev Clin Immunol 2021;17:169-76. [Crossref] [PubMed]
- Torres LK, Pickkers P, van der Poll T. Sepsis-Induced Immunosuppression. Annu Rev Physiol 2022;84:157-81. [Crossref] [PubMed]
- Vázquez AC, Arriaga-Pizano L, Ferat-Osorio E. Cellular Markers of Immunosuppression in Sepsis. Arch Med Res 2021;52:828-35. [Crossref] [PubMed]
- Conway-Morris A, Wilson J, Shankar-Hari M. Immune Activation in Sepsis. Crit Care Clin 2018;34:29-42. [Crossref] [PubMed]
- Mira JC, Gentile LF, Mathias BJ, et al. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med 2017;45:253-62. [Crossref] [PubMed]
- Zhao HQ, Li WM, Lu ZQ, et al. The growing spectrum of anti-inflammatory interleukins and their potential roles in the development of sepsis. J Interferon Cytokine Res 2015;35:242-51. [Crossref] [PubMed]
- Molnár L, Fülesdi B, Németh N, et al. Sepsis-associated encephalopathy: A review of literature. Neurol India 2018;66:352-61. [Crossref] [PubMed]
- Shulyatnikova T, Verkhratsky A. Astroglia in Sepsis Associated Encephalopathy. Neurochem Res 2020;45:83-99. [Crossref] [PubMed]
- Bah I, Kumbhare A, Nguyen L, et al. IL-10 induces an immune repressor pathway in sepsis by promoting S100A9 nuclear localization and MDSC development. Cell Immunol 2018;332:32-8. [Crossref] [PubMed]
- Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol 2019;44:101344. [Crossref] [PubMed]
- Poujol F, Monneret G, Gallet-Gorius E, et al. Ex vivo Stimulation of Lymphocytes with IL-10 Mimics Sepsis-Induced Intrinsic T-Cell Alterations. Immunol Invest 2018;47:154-68. [Crossref] [PubMed]
- Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev 2018;281:179-90. [Crossref] [PubMed]
- Wu C, Ma J, Yang H, et al. Interleukin-37 as a biomarker of mortality risk in patients with sepsis. J Infect 2021;82:346-54. [Crossref] [PubMed]
- Ge Y, Huang M, Yao YM. Recent advances in the biology of IL-1 family cytokines and their potential roles in development of sepsis. Cytokine Growth Factor Rev 2019;45:24-34. [Crossref] [PubMed]
- Abe A, Yamada H. Harmol induces apoptosis by caspase-8 activation independently of Fas/Fas ligand interaction in human lung carcinoma H596 cells. Anticancer Drugs 2009;20:373-81. [Crossref] [PubMed]
- Girardot T, Rimmelé T, Venet F, et al. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2017;22:295-305. [Crossref] [PubMed]
- Su LJ, Zhang JH, Gomez H, et al. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev 2019;2019:5080843. [Crossref] [PubMed]
- Luan YY, Yin CF, Qin QH, et al. Effect of Regulatory T Cells on Promoting Apoptosis of T Lymphocyte and Its Regulatory Mechanism in Sepsis. J Interferon Cytokine Res 2015;35:969-80. [Crossref] [PubMed]
- Andersson G. Evolution of the human HLA-DR region. Front Biosci 1998;3:d739-45. [Crossref] [PubMed]
- Zhuang Y, Peng H, Chen Y, et al. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front Biosci (Landmark Ed) 2017;22:1344-54. [Crossref] [PubMed]
- Winkler MS, Rissiek A, Priefler M, et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PLoS One 2017;12:e0182427. [Crossref] [PubMed]
- Zhou M, Yang WL, Aziz M, et al. Therapeutic effect of human ghrelin and growth hormone: Attenuation of immunosuppression in septic aged rats. Biochim Biophys Acta Mol Basis Dis 2017;1863:2584-93. [Crossref] [PubMed]
- Sun L, Ma W, Gao W, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis 2019;10:542. [Crossref] [PubMed]
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017;277:61-75. [Crossref] [PubMed]
- Wang YC, Liu QX, Liu T, et al. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells predicts the development of sepsis in severe trauma patients: A prospective observational study. Medicine (Baltimore) 2018;97:e9859. [Crossref] [PubMed]
- Shao R, Fang Y, Yu H, et al. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care 2016;20:124. [Crossref] [PubMed]
- Patera AC, Drewry AM, Chang K, et al. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol 2016;100:1239-54. [Crossref] [PubMed]
- Huang SJ, Ai T, Hu H, et al. Immunotherapy for Sepsis Induced by Infections: Clinical Evidence and Potential Targets. Discov Med 2022;34:83-95. [PubMed]
(English Language Editor: L. Huleatt)











