
Revisit of the CatLet (Hexu) angiographic scoring system: a narrative review
Introduction
We have recently developed the Coronary Artery Tree description and Lesion EvaluaTion (CatLet or Hexu, invented by He and Xu) angiographic scoring system based on the 17-myocardial segmental model, law of competitive blood supply, and law of flow conservation (available at www.catletscore.com) (1). Our preliminary studies have demonstrated that this novel angiographic scoring system, accommodating the coronary anatomy in its diversity, can be utilized to predict clinical outcomes for patients with acute myocardial infarction, with a high reproducibility (2-4). Over the past two years, the principles underlying this novel angiographic scoring system do not materially change although slight adjustments have really happened: (I) the short axis of the left ventricle (LV) at the basal level is used to characterize the six types of right coronary artery (RCA) size, which is more understandable and reproducible; (II) segments marked with ’X and ’S have a unified preset difference of one segment as adopted in the characterization of left anterior descending artery (LAD) length; (III) segments marked with ’+ have been added to explain the rare variability in the left circumflex (LCX) or in the posterolateral vessels (PLVs) in cases with posterior descending artery (PDA) only, small RCA, large RCA or super RCA. The CatLet or Hexu angiographic scoring system strictly follows the law of flow conservation in weighting assignment, and the lesion scoring correction has been additionally emphasized and detailed. Given these adjustments and the scoring experience gained in daily use, we think that it is necessary to elaborate on these points so that readers with interest are capable to better use this CatLet or Hexu angiographic scoring system both in clinical practice and in scientific research. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1642/rc).
Methods
A thorough search has been done in the PubMed database using the free text terms “CatLet” AND “angiographic” on September 30th, 2022. Table 1 listed the search strategy for the possible publications on the CatLet angiographic scoring system. RCA, LAD, and LCX compete each other to ensure the blood supply to the whole heart although they vary in size among individuals. We have believed that all this variability among populations should be a continuous spectrum with two extremes at either side in terms of RCA, LCX, or LAD size. Semi-quantification of this variability is helpful for selection of revascularization strategy and risk-stratification for patients with coronary artery disease (CAD).
Table 1
| Items | Specification |
|---|---|
| Date of search | 2022/09/30 |
| Databases and other sources searched | PubMed |
| Search terms used | “CatLet” AND “angiographic” |
| Timeframe | 2019/1/1 to 2022/09/30 |
| Inclusion and exclusion criteria | Without any restrictions |
| Selection process | He YM did the search |
LAD and RCA have relatively consistent running courses and distributing territories: LAD usually runs in the anterior inter-ventricular sulcus with diagonals distributing over the anterior or anterolateral walls; RCA usually gives rise to PDA and PLVs to supply blood to the inferoseptal and the inferior walls. The running courses and distributing territories of LCX vary hugely and are difficult to be defined. LCX may give rise to obtuse marginal (OM) branches to compete with diagonals (Dx) or give rise to both OM and PLVs to simultaneously compete with diagonals and the PLVs arising from RCA to supply blood to the left heart. LCX size can thus be indirectly inferred through the diagonals and the PLVs off RCA (competitive blood supply). In other words, both LAD and RCA have already provided sufficient information on the anatomy, distribution, and blood supply of the entire coronary artery circulation even without knowledge of LCX size in a normal heart.
Key contents and findings: reclassification of RCA
RCA is traditionally dichotomized into right or left dominance although its size varies among individuals. As mentioned above, RCA should also be a continuous spectrum with two extreme scenarios at either side: a very small RCA only to not supply blood to the left ventricle (LV) at all or a very large RCA to supply blood to a large portion of the LV with a resultantly small LCX. For a right dominant heart, RCA runs in the left atrioventricular sulcus (LAVS) beyond the crux cordis, and its size is closely associated with its distribution in the LAVS. The further RCA runs in the LAVS, the larger RCA is.
Based on the 17-myocardial segment model and its recommended assignment of 5 segments to RCA territory, we will define the RCA size for this recommendation on the cineangiogram, and further characterize the RCA’s variability (5). In the 17-myocardial segmental model, the LV is divided into equal thirds perpendicular to the long axis of the heart. This will generate three circular basal, mid-cavity, and apical short axis slices of the LV. With regard to the circumferential location, the six segments of 60° each are divided for the basal and mid-cavity slices, respectively, and four segments of 90° each are divided for the apical slice, where RCA supplies five segments: 3, 4, 9, 10, and 15 according to the statement. Specifically, RCA divides into PDA and PLVs, supplying the two inferoseptal segments (segments 3 and 9) and the three inferior segments (segments 4, 10, and 15), respectively (5). We now focus on the basal short axis slice of the LV and its six segments, which will be approximated as a circle to be analyzed as shown in Figure 1A-1C. Coronary arteries distributing over the inferior segments are projected on the diameter of the circle and cover roughly 1/2 of the diameter (2πr/6≈1/2 diameter). Thus, this distribution of RCA covering roughly 1/2 of the diameter is in theory the recommended RCA size on the cineangiograms if we know the diameter of the basal short axis slice of the LV.
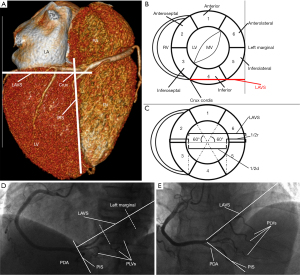
On the cineangiograms, distal PDA usually runs in the posterior inter-ventricular sulcus (PIS), and roughly indicates the long axis of the left heart, and the main PLV (not its branches) running in the LAVS and its extension line corresponds to the basal short axis of the LV in the view of left anterior oblique (LAO) 45°/cranial (CRA) 0°. The length of LAVS starts from the crux cordis (not the origin of PDA) and ends at the left heart margin, which is roughly 3/4 of the diameter of the basal short axis slice of the LV. Therefore, RCA covering roughly 2/3 of LAVS (1/2 of the diameter) represents the recommended RCA size on the cineangiogram as shown in Figure 1D,1E, which is defined as average RCA. We now can further characterize the different RCA sizes based on this average RCA with a preset difference of 1.5 segments.
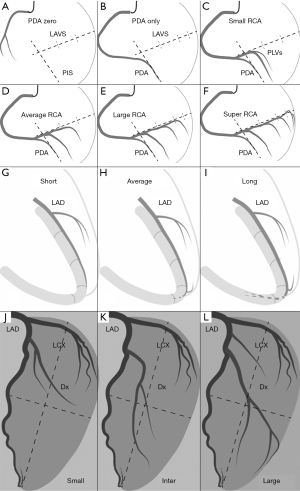
- PDA zero: (left dominance): RCA gives no rise to any branches to supply the LV and the entire LV is supplied by LCA as shown in Figure 2A;
- PDA only: RCA gives rise to an only PDA to supply the inferior septum as shown in Figure 2B, with two segments in total;
- Small RCA: in the view of LAO 45°/CRA 0°, PLVs off RCA distribute around the level of the first third of the LAVS to supply a portion of the inferior segments as shown in Figure 2C, with 3.5 segments in total;
- Average RCA: in the view of LAO 45°/CRA 0°, PLVs off RCA distribute around the level of 2/3 of the LAVS to supply the entire inferior segments as shown in Figure 2D, with 5 segments in total;
- Large RCA: in the view of LAO 45°/CRA 0°, PLVs off RCA distribute around the level of the earlier 1/2 of the last third of the LAVS to supply the entire inferior wall and a portion of the inferolateral wall as well as shown in Figure 2E, with 6.5 segments in total;
- Super RCA: in the view of LAO 45°/CRA 0°, PLVs off RCA distribute around the level of the later 1/2 of the last third of the LAVS to supply the entire inferior segments, inferolateral segments, and a portion of the anterolateral segments as shown in Figure 2F, with 8 segments in total.
Reclassification of LAD or Dx
Reclassification of LAD or Dx has been described in detail elsewhere (1). In brief, in the view of RAO 30°/cranial 20°, according to the running course of the LAD in the inter-ventricular sulcus (anterior or posterior), three types of LAD (Figure 2G-2I) are classified with a preset difference of one segment: short LAD is defined as its termination before the apex segment with 3 segments supplied in total; average LAD is defined as its supplying blood to the apex segment with 4 segments in total; and long LAD is defined as its running further in the posterior inter-ventricular sulcus after turning around the apical notch and supplying blood to a portion of inferoseptum, with 5 segments in total. In the view of LAO 40°/CRA 20°, the anterior wall of the LV, outlined by the LAD and the left margin of the heart (not the LCX), is divided into four quadrants via the long axis line and the short axis line of the LV perpendicularly intersected at their midpoints. According to the distribution of Dx over the four quadrants, three types of diagonals (Figure 2J-2L) are classified with a preset difference of one segment: small Dx is defined as its distributing over ≤2 quadrants, with 2 segments supplied in total; inter. Dx is defined as its distributing over 3 quadrants, with 3 segments supplied in total; and large Dx is defined as its distributing over ≥4 quadrants (at least a half of the quadrant four occupied), with 4 segments supplied in total. Six types of RCA, three types of LAD, and three types of Dx together result in 54 patterns of coronary circulation, which, in combination with the segmentation for the coronary trees, will be used to account for the variability in the coronary anatomy (1).
Weighting assignment to coronary segments for the 54 patterns of coronary circulation in a regular scheme or in an alternative scheme
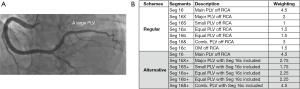
Weighting assignment has been slightly adjusted as compared with our previously reported one (1). As in the reclassification of LAD length, segments marked with ’X or ’S have also a unified preset difference of one segment. Characterization of RCA size remains unchanged with a preset difference of 1.5 segments. In most Large RCA cases, the regular weighting assignment to PLVs works well. However, rarely in Large RCA case, does this regular scheme not work well because of the presence of a particularly large PLV while an alternative scheme works well as shown in Figure 3. This is also the case in the PDA only, Small RCA, or Super RCA, where segments marked with ’+ indicate their weighting reassignments with additional segments included (e.g., seg 16c or seg 13). The table online (available online: https://cdn.amegroups.cn/static/public/jtd-22-1642-1.xlsx) showed weighting assignment to coronary segments for the 54 patterns of coronary circulation.
Lesion scoring and its correction as appropriate in the CatLet or Hexu score calculation
A lesion or multiple lesions are defined as in the SYNTAX score (6). A lesion is defined as ≥50% diameter stenosis by visual assessment in vessels ≥1.5 mm in diameter; lesions more than three reference diameter apart are considered multiple lesions. The CatLet or Hexu score calculator is accessible at www.catletscore.com. Its use has been described in full elsewhere (1). Figure 4 briefly demonstrates the scoring process. Click the CatLet or Hexu score calculator and dialog boxes will pop up. After filling in the ID and name boxes, select a coronary dominance for RCA, Dx, and LAD, respectively, which will define a personalized coronary circulation pattern for further evaluation of the lesions. Coronary segments and their corresponding weightings for this coronary circulation pattern will pop up, and a lesion-based evaluation will be performed for selection of involved segments. Nine types of lesion will pop up for selection. Subsequently, the adverse angiographic characteristics (culprit vessel, bifurcation, trifurcation, calcification, etc.) pertinent to the lesion and the approach to the lesion will require to be answered. After completion of the lesion evaluation, you can finish its evaluation or add another lesion for repeat evaluation. Finally, the CatLet or Hexu angiographic scoring system will explain native the anatomy in the coronary trees. All the information collected can be copied and pasted into the EXCEL sheet for further approach. The lesion on the segment 6 (Figure 5) and its pertinent adverse characteristics are evaluated as an example using the CatLet or Hexu angiographic scoring system as shown in Table 2, and multiple lesion scores are added to derive the total score if applicable.
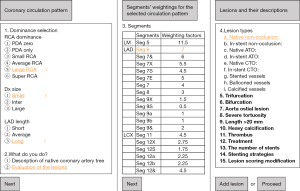
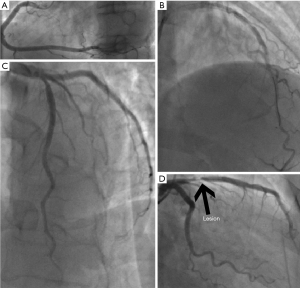
Table 2
| ID | Name | Culprit lesion | Lesion number | Item name | Description | Values |
|---|---|---|---|---|---|---|
| 6666 | John | – | – | RCA dominance | – | Large RCA |
| 6666 | John | – | – | LAD length | – | Long |
| 6666 | John | – | – | Dx size | – | Small |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | 7 * 2 = | 14 |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Lesion types | Native non-occlusion |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Trifurcation | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Bifurcation | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Aorto-ostial lesion | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Severe tortuosity | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Length >20 mm | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Heavy calcification | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Thrombus | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Treatment | Untouched |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Lesion scoring modification | No |
| 6666 | John | Culprit | Lesion 1 | Seg 6 | Subtotal score 1 | 14 |
| 6666 | John | – | – | Stenosis lesion number | – | 1 |
| 6666 | John | – | – | Total score | – | 14 |
| 6666 | John | – | – | Calcification number on lesions | – | 0 |
| 6666 | John | – | – | Current stent number | – | 0 |
Seg, segment; CatLet, Coronary Artery Tree description and Lesion EvaluaTion; RCA, right coronary artery; LAD, left anterior descending artery; Dx, diagonals.
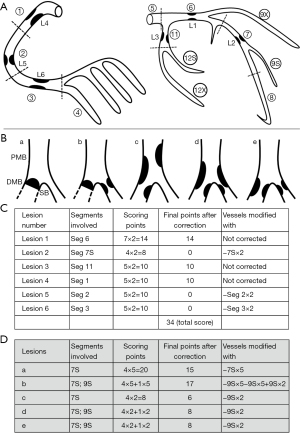
According to the law of flow conservation, a parent vessel equals the sum of its daughter vessels in terms of coronary flow. The weighting assignment in the CatLet or Hexu score strictly follows this law (1). Therefore, the weighting for a parent vessel has already covered the ones for its daughter vessels. A lesion scoring on a daughter vessel must be corrected as zero if a lesion scoring on its parent vessel has already been done as shown in Figure 6. It thus makes sense that the scoring points are up to 34 points for a heart with non-occlusive lesions and 85 points for a heart with occlusive lesions.
Available studies on the CatLet or Hexu angiographic scoring system and its future study directions
Since our first introduction of the CatLet or Hexu angiographic scoring system in 2019, series of studies on this novel scoring system have been published, where its utilities have been validated in outcome predictions for patients with acute myocardial infarction. The first validation study on the CatLet or Hexu angiographic scoring consecutively enrolled 308 patients with acute myocardial infarction undergoing primary PCI (ChiCTR-POC-17013536). Primary endpoint was major adverse cardiac or cerebrovascular events (MACCEs) at 4.3 years. The CatLet score was tertile partitioned: CatLetlow ≤14 (n=124), CatLetmid 15–21 (n=82) and CatLettop ≥22 (n=102). Multivariable-adjusted analysis revealed that the CatLet or Hexu score predicted MACCEs at 4.3-year follow-up; more importantly, the CatLet score performed better than the SYNTAX score in this head-to-head comparison study (3). The post hoc study of this first validation study has demonstrated that age, serum creatinine, and left ventricular ejection fraction had added values in predicting the clinical outcomes as compared with the stand-alone CatLet score (2). The extended CatLet or Hexu validation study, registered number being ChiCTR2000033730, consecutively enrolled 1,018 patients with acute myocardial infarction. These patients arrived at our hospital later than >12 h and received the elective PCI. The primary endpoint was major adverse cardiac events (MACEs), defined as a composite of non-fatal myocardial infarction, cardiac death, and ischemia-driven revascularization. The CatLet or Hexu score was also tertile partitioned: CatLetlow ≤12, CatLetmid 3–18, and CatLettop ≥19. The CatLet or Hexu score-MACEs association was examined using the Logistic model and Kaplan-Meiere curve. In the Kaplan-Meiere analysis, patients in the CatLetmid and in the CatLettop were associated with hazard ratios (95% CI) 1.68 (1.15–2.44)-fold, and 3.59 (2.56–5.03)-fold increased risk of MACEs, respectively. The discriminations and calibrations of the CatLet or Hexu score for MACEs were also good (7). We also investigated the reproducibility of the CatLet or Hexu angiographic scoring system. The weighted kappa values (95% CI) for the intra- and inter-observer reproducibility of the CatLet Score were 0.82 (0.59–1.00, Z=7.23, P<0.001) and 0.86 (0.54–1.00, Z=5.20, P<0.001), respectively, according to the tertile analysis (14, 15–22, >22) (4). The CatLet or Hexu angiographic scoring system will be further validated in different populations with coronary artery disease. Competitive blood supply to the left ventricle has formed the basis of derivation of the CatLet or Hexu angiographic scoring system. It is thus perhaps useful to diagnose patients with congenital abnormal development in coronary arteries. Fraction flow reserve (FFR) guided-PCI has been recommended by guidelines with Class I or IIa and level of evidence A (8,9). Prior studies showed that FFR values measured were not only associated with the degree of stenosis of a coronary artery, but also with the subtended myocardial territory by the diseased coronary artery (10,11). The CatLet or Hexu angiographic scoring system is also capable to semi-quantify the subtended myocardial territory by the diseased coronary artery. The CatLet score-FFR association is thus deserving of being explored (ChiCTR2300068351); another prospective study on the CatLet score and myocardial strain has also been underway (ChiCTR2300068814), both studies being registered on https://www.chictr.org.cn/.
Limitations
Several major limitations should be considered before we use this novel angiographic scoring system: (I) the CatLet or Hexu angiographic scoring system has been developed a priori. Therefore, any extrapolations on this scoring system should be reconsidered before we can validate it; (II) in the CatLet or Hexu angiographic scoring system, the degree of stenosis is dichotomized at 50% diameter stenosis, failing to reflect the different stenosis degrees of a coronary artery. The Gensini score has accounted for the different stenosis degrees of a coronary artery. Therefore, the CatLet or Hexu angiographic scoring system considering the different degrees of a stenosis can be refined in outcome predictions; (III) finally, all the LAD, LCX or RCA can supply blood to the apex segment, where the law of competitive blood supply does not kind of work.
Conclusions
An adjusted method for characterization of RCA in the view of LAO 45°/CRA 0° is more understandable and easier in its use. A lesion score on a daughter vessel is required to be corrected after its parent vessel has already been scored. These adjustments and scoring experience gained on the CatLet or Hexu angiographic scoring system will help to boost its use in cardiovascular field. The utility of this novel angiographic scoring system has been preliminarily validated with respect to outcome predictions and its unreclaimed fields are noteworthy in the future study.
Acknowledgments
Funding: This work was supported by Sci-Tech Supporting Program of Jiangsu Commission of Health (M2021019) and Medical Sci-Tech innovation Program for Medical Care of Suzhou City (SKY2021005).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1642/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1642/coif). All authors report that this work was supported by Sci-Tech Supporting Program of Jiangsu Commission of Health (M2021019) and Medical Sci-Tech innovation Program for Medical Care of Suzhou City (SKY2021005). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu MX, Teng RL, Ruddy TD, et al. The CatLet score: a new coronary angiographic scoring tool accommodating the variable coronary anatomy for the first time. J Thorac Dis 2019;11:5199-209. [Crossref] [PubMed]
- Teng RL, Liu M, Sun BC, et al. Age, Serum Creatinine, and Left Ventricular Ejection Fraction Improved the Performance of the CatLet Angiographic Scoring System in Terms of Outcome Predictions for Patients with Acute Myocardial Infarction: A Median 4.3-Year Follow-Up Study. Cardiology 2021;146:690-7. [Crossref] [PubMed]
- Xu MX, Ruddy TD, Schoenhagen P, et al. The CatLet score and outcome prediction in acute myocardial infarction for patients undergoing primary percutaneous intervention: A proof-of-concept study. Catheter Cardiovasc Interv 2020;96:E220-9. [Crossref] [PubMed]
- Liu JM, He Y, Teng RL, et al. Inter- and intra-observer variability for the assessment of coronary artery tree description and lesion EvaluaTion (CatLet©) angiographic scoring system in patients with acute myocardial infarction. Chin Med J (Engl) 2020;134:425-30. [Crossref] [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42. [Crossref] [PubMed]
- Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219-27. [PubMed]
- Wang H, He Y, Fan JL, et al. The predictive value of CatLet© angiographic scoring system for long-term prognosis in patients with acute myocardial infarction presenting > 12 h after symptom onset. Front Cardiovasc Med 2022;9:943229. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Writing Committee Members. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:197-215. [PubMed]
- Iqbal MB, Shah N, Khan M, et al. Reduction in myocardial perfusion territory and its effect on the physiological severity of a coronary stenosis. Circ Cardiovasc Interv 2010;3:89-90. [Crossref] [PubMed]
- De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart 2008;94:949-59. [Crossref] [PubMed]


