
Predictive value of clinical features for anti-reflux therapy response in suspected gastroesophageal reflux-induced chronic cough
Highlight box
Key findings
• Over half of the suspected gastroesophageal reflux-induced cough patients benefited from anti-reflux therapy. A few clinical features rather than reflux-related symptoms might indicate a response to anti-reflux treatment.
What is known and what is new?
• Among patients with chronic cough, anti-reflux treatment is recommended in patients with typical reflux-related symptoms or in who had no evidence of other common causes of chronic cough. It remains unknown whether reflux-related symptoms and other clinical features could predict the response to anti-reflux therapy in suspected GERC patients.
• A few clinical features rather than reflux-related symptoms might indicate a response to anti-reflux treatment.
What is the implication, and what should change now?
• Nasal itching, tickle in the throat, pharyngeal foreign body sensation and sensitivity to at least one cough trigger could be used as a screening tool to guide the anti-reflux therapy.
Introduction
The common causes of chronic cough include cough variant asthma (CVA), eosinophilic bronchitis (EB), upper airway cough syndrome (UACS), and gastroesophageal reflux-induced cough (GERC). GERC accounts for 4.6–41% of chronic cough (1-3) and impaired significantly the quality of life (4,5). Etiological diagnosis of chronic cough is a key step to a favorable treatment response. It requires multiple investigations, such as spirometry, bronchial challenge, induced sputum test for differential cells, and 24-hour esophageal pH value monitoring. These investigations, especially 24-hour esophageal pH value monitoring are time-consuming, expensive, and unavailable in the primary care setting. Our previous study found that a few clinical features could indicate a single common cause of chronic cough, such as nocturnal cough for CVA, postnasal dripping and history of sinusitis for UACS, heartburn, belching, acid regurgitation and cough after meals for GERC (6). Empiric therapy based on clinical characteristics is recommended in primary care (7).
According to the American College of Chest Physicians (ACCP) Clinical Practice Guideline for cough due to gastroesophageal reflux disease (GERD), GERC should be considered if chronic cough patients present with typical reflux-related symptoms, or had no evidence of other common causes of chronic cough (2,8). For these patients with suspected GERC, anti-reflux treatment is recommended (2,8). However, this recommendation was based on observational studies with a small sample and a low level of evidence (9,10). A previous study found that only 35% of patients with chronic cough responded favorably to proton pump inhibitor (PPI) treatment after excluding asthma (11).
For patients with chronic cough, reflux-related symptoms may indicate a diagnosis of GERC to some extent, in comparison with other common causes of chronic cough (6). However, it remains unknown whether reflux-related symptoms and other clinical features could predict the response to anti-reflux therapy in suspected GERC patients. It would be meaningful to identify a few clinical features for predicting anti-reflux therapy response. Therefore, we conducted this retrospective study to investigate the relationship between the clinical features and the response to anti-reflux therapy in suspected GERC patients from a chronic cough database with standard case report form. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1046/rc).
Methods
This was a retrospective observational study. Data were collected from a chronic cough database in the First Affiliated Hospital of Guangzhou Medical University from 2006 to 2021. We enrolled the chronic cough patients who presented cough as the sole or predominant symptom lasting at least 8 weeks with normal chest X-ray. A subset of patients come from our previous prospective studies (12,13). The standard case report form was used to record demographics, clinical features, laboratory results, primary diagnosis, response to therapy, and final diagnosis as described previously. Spirometry, bronchial challenge, and induced sputum test for differential cells were performed in all patients, and 24-hour esophageal pH value monitoring was performed in a subset of patients (2,14). DeMeester score and symptom association probability (SAP) were recorded in the chronic cough database. Pathological reflux was defined as a distal DeMeester score ≥12.7 or distal SAP ≥95% (15).
The inclusion criteria for patients with suspected GERC were as follows: (I) presence of typical reflux-related symptoms, including acid regurgitation, belching, heartburn, or abnormal 24-hour esophageal pH value monitoring results; or (II) no evidence of CVA, EB, UACS, atopic cough, and other potential causes, and no response to treatment directed to these potential causes of chronic cough; (III) anti-reflux therapy for at least 2 weeks, PPIs (omeprazole or esomeprazole, 20 mg, bid) plus prokinetic agents (domperidone, 10 mg, tid or mosapride, 5 mg, tid) were administered in all suspected GERC patients. We excluded the patients who underwent anti-reflux therapy combined with other therapy and patients with multi causes of chronic cough in this study.
The patients with suspected GERC were divided into responders and non-responders according to the response to anti-reflux treatment. Responders were defined as patients who had a self-reported resolution of cough after anti-reflux treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020150). All patients gave informed consent for their data to be analyzed.
Statistical analyses
Data were expressed as frequency (percentage), mean ± SD, or median (interquartile range). Statistical comparisons between groups were performed with unpaired Student’s t-test for normally distributed data, Mann-Whitney U tests for skewed data, and χ2 tests or Fisher exact tests for categorical variables. A Cox regression test was used to identify the variables that were independent predictors of the response to anti-reflux treatment. Variables whose P value <0.1 in univariable models was put into the multivariable model. Multiple Cox regression analysis was conducted with the method of forward stepwise (likelihood ratio). For those clinical features that indicated response to anti-reflux therapy (P<0.05), sensitivities, specificities, and area under curve (AUC) were calculated. For patients who did not complete 24-hour esophageal pH value monitoring, we handled missing data as missing without data imputation. Rstudio (Version 1.4.1717.0; Free Software Foundation, Inc., Boston, MA, USA) was used for statistical analyses.
Results
The relationship between clinical features and response to anti-reflux therapy
A total of 241 patients with suspected GERC were enrolled. Among this group of eligible patients, 111 (46.1%) patients were female and the median age was 37.0 [interquartile range (IQR), 30.0–49.0] years. The median course of the disease was 30.0 (IQR, 12.0–72.0) months.
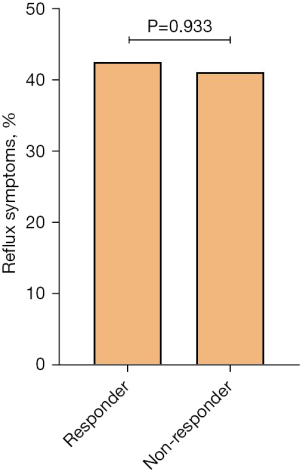
Among 241 patients evaluated in this study, cough resolved in 146 (60.6%) patients. The demographic characteristics of responders and non-responders are shown in Table 1. Responders showed a significantly higher proportion of nasal itching (21.2% vs. 8.4%; P=0.014) and tickle in the throat (51.4% vs. 35.8%; P=0.025) than non-responders whereas the proportion of pharyngeal foreign body sensation (32.9% vs. 54.7%; P=0.001) was significantly lower in responders. One hundred and four (71.2%) responders presented at least one of the reflux-associated symptoms (acid regurgitation, nausea, belching, heartburn, and chest tightness) while 56 (58.9%) non-responders showed at least one of them (P>0.05). The frequency of trigger-eating (32.2% vs. 34.7%), acid regurgitation (36.3% vs. 36.8%), nausea (28.8% vs. 20.0%), belching (39.7% vs. 27.4%), heartburn (19.2% vs. 14.7%), chest tightness (31.5% vs. 31.6%), the presence of acid regurgitation or heartburn (42.5% vs. 41.1%), and the number of reflux-related symptoms did not differ between responders and non-responders (P>0.05) (Table 1, Figure 1).
Table 1
| Variables | Total (n=241) | Responder (n=146) | Non-responder (n=95) | P value |
|---|---|---|---|---|
| Female, n (%) | 111 (46.1) | 67 (45.9) | 44 (46.3) | 1.000 |
| Age (years), median (Q1, Q3) | 37.0 (30.0, 49.0) | 38.0 (30.0, 50.0) | 36.0 (29.5, 47.0) | 0.740 |
| Course of disease (months), median (Q1, Q3) | 30.0 (12.0, 72.0) | 24.0 (12.0, 84.0) | 36.0 (12.0, 72.0) | 0.771 |
| VAS at admission, median (Q1, Q3) | 60.0 (50.0, 80.0) | 60.0 (50.0, 80.0) | 60.0 (50.0, 75.0) | 0.631 |
| Cough phase, n (%) | ||||
| Cough in daytime | 225 (93.4) | 135 (92.5) | 90 (94.7) | 0.669 |
| Cough after sleep | 67 (27.8) | 43 (29.5) | 24 (25.3) | 0.574 |
| Cough after waking up | 95 (39.4) | 55 (37.7) | 40 (42.1) | 0.580 |
| Nonproductive cough, n (%) | 177 (73.4) | 111 (76.0) | 66 (69.5) | 0.329 |
| Sensitive to at least one cough trigger, n (%) | 228 (95.8) | 135 (93.8) | 93 (98.9) | 0.094 |
| Trigger-eating, n (%) | 80 (33.2) | 47 (32.2) | 33 (34.7) | 0.787 |
| After meal | 55 (22.8) | 32 (21.9) | 23 (24.2) | 0.797 |
| During meal | 31 (12.9) | 21 (14.4) | 10 (10.5) | 0.498 |
| Reflux-related symptoms, n (%) | 160 (66.4) | 104 (71.2) | 56 (58.9) | 0.067 |
| Acid regurgitation | 88 (36.5) | 53 (36.3) | 35 (36.8) | 1.000 |
| Nausea | 61 (25.3) | 42 (28.8) | 19 (20.0) | 0.168 |
| Belching | 84 (34.9) | 58 (39.7) | 26 (27.4) | 0.067 |
| Heartburn | 42 (17.4) | 28 (19.2) | 14 (14.7) | 0.475 |
| Chest tightness | 76 (31.5) | 46 (31.5) | 30 (31.6) | 1.000 |
| Acid regurgitation or Heartburn | 101 (41.9) | 62 (42.5) | 39 (41.1) | 0.933 |
| Acid regurgitation or Belching or Heartburn | 129 (53.5) | 83 (56.8) | 46 (48.4) | 0.250 |
| Number of reflux-related symptoms, n (%) | 0.493 | |||
| 0–2 | 186 (77.2) | 110 (75.3) | 76 (80.0) | |
| 3–5 | 55 (22.8) | 36 (24.7) | 19 (20.0) | |
| Nasal symptoms, n (%) | 121 (50.2) | 70 (47.9) | 51 (53.7) | 0.460 |
| Sneezing | 53 (22.0) | 33 (22.6) | 20 (21.1) | 0.901 |
| Nasal itching | 39 (16.2) | 31 (21.2) | 8 (8.4) | 0.014 |
| Runny nose | 50 (20.7) | 30 (20.5) | 20 (21.1) | 1.000 |
| Postnasal dripping | 44 (18.3) | 28 (19.2) | 16 (16.8) | 0.773 |
| Nasal congestion | 65 (27.0) | 38 (26.0) | 27 (28.4) | 0.794 |
| Shortness of breath, n (%) | 51 (21.2) | 30 (20.5) | 21 (22.1) | 0.898 |
| Pharyngeal symptoms, n (%) | 211 (87.6) | 129 (88.4) | 82 (86.3) | 0.788 |
| Tickle in the throat | 109 (45.2) | 75 (51.4) | 34 (35.8) | 0.025 |
| Tickle below the throat | 49 (20.3) | 31 (21.2) | 18 (18.9) | 0.789 |
| Pharyngeal foreign body sensation | 100 (41.5) | 48 (32.9) | 52 (54.7) | 0.001 |
| Frequent throat clearing | 109 (45.2) | 66 (45.2) | 43 (45.3) | 1.000 |
| Mucus adhesion to the throat | 87 (36.1) | 52 (35.6) | 35 (36.8) | 0.955 |
| Medical history, n (%) | ||||
| Rhinitis | 41 (17.0) | 31 (21.2) | 10 (10.5) | 0.047 |
| Sinusitis | 33 (13.7) | 21 (14.4) | 12 (12.6) | 0.845 |
| Gastrointestinal disorders | 61 (25.4) | 40 (27.6) | 21 (22.1) | 0.422 |
| Hypertension | 11 (6.5) | 10 (8.8) | 1 (1.8) | 0.105 |
| 24-hour pH-metry | n=137 | n=94 | n=43 | |
| Distal DeMeester score, median (Q1, Q3) | 12.5 (4.7, 23.0) | 14.6 (5.2, 24.8) | 10.4 (2.9, 17.9) | 0.058 |
| Distal DeMeester score ≥12.7, n (%) | 67 (48.9) | 51 (54.3) | 16 (37.2) | 0.095 |
| Distal SAP, median (Q1, Q3) | 88.8 (31.0, 99.0) | 87.8 (38.8, 99.0) | 92.0 (0.0, 99.0) | 0.890 |
| Distal SAP ≥0.95, n (%) | 52 (38.0) | 36 (38.3) | 16 (37.2) | 1.000 |
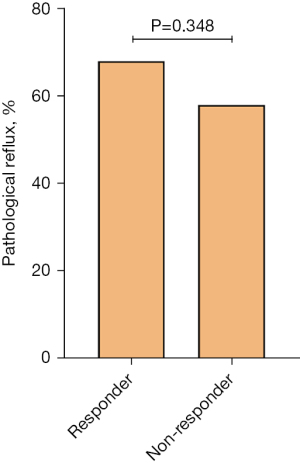
| Abnormal pH results, n (%) | 89 (65.0) | 64 (68.1) | 25 (58.1) | 0.348 |
Trigger-eating, cough occurs after meal or during meal. Q1, the first quartile; Q3, the third quartile; VAS, a 10 cm scale to evaluate severity of cough, with higher score meaning more severe cough; SAP, symptom association probability; VAS, visual analogue scale. Abnormal pH results, distal DeMeester score ≥12.7 or distal SAP ≥95%.
For all patients recruited in this study, the univariate Cox regression analysis indicated that potential factors associated with the response to anti-reflux therapy were reflux-associated symptoms, sensitive to at least one cough trigger, tickle in the throat, pharyngeal foreign body sensation, nasal itching and rhinitis (P<0.1). Multivariate analysis showed that nasal itching [hazard ratio (HR): 1.593, 95% confidence interval (CI): 1.025–2.476, P=0.039] and tickle in the throat (HR: 1.605, 95% CI: 1.152–2.238, P=0.005) were predictive factors for a response to therapy while pharyngeal foreign body sensation (HR: 0.499, 95% CI: 0.346–0.720, P<0.001) and sensitive to at least one cough trigger (including dust, cooking fume, cold air, supine position, cigarette smoke, exercise, talking, alcohol, eating or others) (HR: 0.480, 95% CI: 0.237–0.973, P=0.042) were associated with no response to anti-reflux therapy (Table 2).
Table 2
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | 1.080 | 0.779–1.495 | 0.645 | 1.317 | 0.910–1.907 | 0.144 | |
| Age | 1.005 | 0.992–1.018 | 0.465 | 1.001 | 0.987–1.015 | 0.908 | |
| Cough triggers* | 0.555 | 0.282–1.090 | 0.087 | 0.480 | 0.237–0.973 | 0.042 | |
| Reflux-related symptoms | 1.398 | 0.977–2.001 | 0.067 | 1.391 | 0.962–2.011 | 0.079 | |
| Tickle in the throat | 1.646 | 1.189–2.281 | 0.003 | 1.605 | 1.152–2.238 | 0.005 | |
| Pharyngeal foreign body sensation | 0.538 | 0.381–0.761 | <0.001 | 0.499 | 0.346–0.720 | <0.001 | |
| Nasal itching | 1.673 | 1.125–2.488 | 0.011 | 1.593 | 1.025–2.476 | 0.039 | |
| Rhinitis | 1.788 | 1.201–2.660 | 0.004 | 1.457 | 0.933–2.276 | 0.098 | |
*, cough triggers: cough induced by dust, cooking fume, cold air, supine position, cigarette smoke, exercise, talking, alcohol, eating or others. HR, hazard ratio; CI, confidence interval.
The combination of nasal itching, tickle in the throat, pharyngeal foreign body sensation, and sensitive to at least one cough trigger had low sensitivity of 0.497 and specificity of 0.805 to predict the successful response to anti-reflux treatment, with an AUC of 0.713.
The relationship between clinical features and response to anti-reflux therapy in GERC patients with abnormal results of 24-hour pH esophageal monitoring
In 89 patients with abnormal pH value monitoring results, 60 (67.4%) showed a good response to anti-reflux therapy, whereas the remaining 29 (32.6%) reported an unfavorable response. Compared with non-responders, the responders presented a similar frequency of cough trigger and reflux-related symptoms, but a higher proportion of nasal itching (26.7% vs. 3.4%; P=0.020) and tickle in the throat (65.0% vs. 31.0%; P=0.005) (Table 3).
Table 3
| Variables | Total (n=89) | Responder (n=60) | Non-responder (n=29) | P value |
|---|---|---|---|---|
| Female, n (%) | 33 (37.1) | 20 (33.3) | 13 (44.8) | 0.413 |
| Age (years), median (Q1, Q3) | 37.0 (30.0, 50.0) | 36.0 (30.0, 51.5) | 38.0 (33.0, 44.0) | 0.990 |
| Course of disease (months), median (Q1, Q3) | 36.0 (24.0, 96.0) | 36.0 (16.5, 96.0) | 39.0 (24.0, 96.0) | 0.349 |
| VAS at admission, median (Q1, Q3) | 60.0 (50.0, 80.0) | 60.0 (50.0, 80.0) | 60.0 (50.0, 80.0) | 0.833 |
| Cough phase, n (%) | ||||
| Cough in daytime | 81 (91.0) | 56 (93.3) | 25 (86.2) | 0.430 |
| Cough after sleep | 23 (25.8) | 20 (33.3) | 3 (10.3) | 0.039 |
| Cough after waking up | 34 (38.2) | 21 (35.0) | 13 (44.8) | 0.508 |
| Nonproductive cough, n (%) | 66 (74.2) | 46 (76.7) | 20 (69.0) | 0.603 |
| Sensitive to at least one cough trigger, n (%) | 85 (96.6) | 56 (94.9) | 29 (100.0) | 0.548 |
| Trigger-eating, n (%) | 34 (38.2) | 25 (41.7) | 9 (31.0) | 0.462 |
| After meal | 23 (25.8) | 16 (26.7) | 7 (24.1) | 1.000 |
| During meal | 14 (15.7) | 12 (20.0) | 2 (6.9) | 0.133 |
| Reflux-related symptoms, n (%) | 62 (69.7) | 44 (73.3) | 18 (62.1) | 0.402 |
| Acid regurgitation | 42 (47.2) | 30 (50.0) | 12 (41.4) | 0.591 |
| Nausea | 30 (33.7) | 19 (31.7) | 11 (37.9) | 0.729 |
| Belching | 34 (38.2) | 24 (40.0) | 10 (34.5) | 0.788 |
| Heartburn | 21 (23.6) | 15 (25.0) | 6 (20.7) | 0.855 |
| Chest tightness | 27 (30.3) | 20 (33.3) | 7 (24.1) | 0.523 |
| Acid regurgitation or Heartburn | 48 (53.9) | 34 (56.7) | 14 (48.3) | 0.605 |
| Acid regurgitation or Belching or Heartburn | 55 (61.8) | 39 (65.0) | 16 (55.2) | 0.508 |
| Number of reflux-related symptoms, n (%) | 1.000 | |||
| 0–2 | 64 (71.9) | 43 (71.7) | 21 (72.4) | |
| 3–5 | 25 (28.1) | 17 (28.3) | 8 (27.6) | |
| Nasal symptoms, n (%) | 52 (58.4) | 38 (63.3) | 14 (48.3) | 0.262 |
| Sneezing | 27 (30.3) | 21 (35.0) | 6 (20.7) | 0.258 |
| Nasal itching | 17 (19.1) | 16 (26.7) | 1 (3.4) | 0.020 |
| Runny nose | 20 (22.5) | 16 (26.7) | 4 (13.8) | 0.274 |
| Postnasal dripping | 18 (20.2) | 13 (21.7) | 5 (17.2) | 0.837 |
| Nasal congestion | 29 (32.6) | 20 (33.3) | 9 (31.0) | 1.000 |
| Shortness of breath, n (%) | 11 (12.4) | 7 (11.7) | 4 (13.8) | 0.744 |
| Pharyngeal symptoms, n (%) | 78 (87.6) | 52 (86.7) | 26 (89.7) | 1.000 |
| Tickle in the throat | 48 (53.9) | 39 (65.0) | 9 (31.0) | 0.005 |
| Tickle below the throat | 23 (25.8) | 15 (25.0) | 8 (27.6) | 0.998 |
| Pharyngeal foreign body sensation | 24 (27.0) | 12 (20.0) | 12 (41.4) | 0.061 |
| Frequent throat clearing | 40 (44.9) | 24 (40.0) | 16 (55.2) | 0.262 |
| Mucus adhesion to the throat | 26 (29.2) | 15 (25.0) | 11 (37.9) | 0.313 |
| Medical history, n (%) | ||||
| Rhinitis | 15 (16.9) | 15 (25.0) | 0 (0.0) | 0.002 |
| Sinusitis | 9 (10.1) | 7 (11.7) | 2 (6.9) | 0.712 |
| Gastrointestinal disorders | 29 (33.0) | 19 (32.2) | 10 (34.5) | 1.000 |
| Hypertension | 5 (6.5) | 5 (9.3) | 0 (0.0) | 0.314 |
Trigger-eating, cough occurs after meal or during meal. Q1, the first quartile; Q3, the third quartile; VAS, a 10 cm scale to evaluate severity of cough, with higher score meaning more severe cough; VAS, visual analogue scale.
Comparison of 24-hour esophageal pH-value monitor results between responders and non-responders
Among 137 patients who completed 24-hour ambulatory esophageal pH-monitor, 94 (68.6%) showed successful responses to anti-reflux treatment. Distal DeMeester score, [14.6 (5.2, 24.8) vs. 10.4 (2.9, 17.9)], the proportion of patients with distal DeMeester score ≥12.7 (54.3% vs. 37.2%), distal SAP [87.8 (38.8, 99.0) vs. 92.0 (0.0, 99.0)], the proportion of patients with distal SAP ≥0.95 (38.3% vs. 37.2%), and the proportion of pathological reflux results (68.1% vs. 58.1%) did not differ between responders and non-responders (P>0.05) (Table 1, Figure 2).
Discussion
This study investigated the relationship between clinical features and response to anti-reflux therapy in a group of suspected GERC patients. In this study, we found that more than half of patients benefited from anti-reflux therapy in patients who had evidence of reflux (reflux-related symptoms or abnormal results of 24-hour pH esophageal monitoring) or who had no evidences of other common causes of chronic cough (12,15,16). Nasal itching, tickle in the throat, pharyngeal foreign body sensation, and sensitive to cough triggers were associated with anti-reflux therapy response in suspected GERC patients.
In ACCP guidelines on GERC, anti-reflux treatment was recommended to chronic cough patients after other common causes of chronic cough were excluded, even though patients did not have concomitant reflux-related symptoms (2,8). In this study, around one-third of patients did not benefit from anti-reflux therapy. These patients might have reflux symptoms or evidence of reflux, but the reflux might not be the cause of the cough (4). In addition, this ineffectiveness might be attributed to refractory reflux-related cough (6). The proportion of patients with common causes decreased and the proportion of patients with unexplained cough or refractory chronic cough increased in our center in recent years (6) because more chronic cough patients with common causes could be diagnosed and treated in primary care clinics or secondary health care after the promotion of Chinese guidelines on cough. These patients with refractory reflux-related cough might need intensive anti-reflux treatment, such as doubling the dose of PPIs, neuromodulators, or even surgery. In addition, although most patients with suspected GERC would respond to anti-reflux therapy within 2 weeks (17,18), the cough might improve after longer treatment occasionally. We could not rule out that a few patients might need longer-duration treatment to improve their cough.
The relationship between reflux-related symptoms and the anti-reflux therapy efficacy in suspected GERC is still controversial. Previous data found that symptom scores of reflux-associated symptoms were not associated with definite, sustained improvement in cough for >3 months, although the symptom score in this article was not clearly defined (19). In our study, reflux-related symptoms were also not associated with anti-reflux therapy efficacy. However, Xu et al. (20) reported that a comprehensive questionnaire could identify responsiveness to anti-reflux therapy in suspected GERC. This comprehensive questionnaire comprised the severity of reflux-related symptoms, quality of sleep related to symptoms, and additional medication targeting heartburn and/or regurgitation in the preceding week, with a scale ranging from 0 to 3 for each item. An overall score ≥8 indicated a response to anti-reflux treatment for 8 weeks. These results suggested that higher reflux-related symptom scores might indicate a better response to anti-reflux therapy for GERC.
It is surprising that higher proportions of nasal itching and tickle in the throat were found in responders to anti-reflux treatment. It is well known that a variety of extraesophageal symptoms could be caused by direct stimulation of the pharynx, larynx, or airway or indirect stimulation of neurogenic inflammation. Laryngopharyngeal reflux may induce chronic laryngitis or laryngopharyngitis (21), which often presents with throat clearing, pharyngeal foreign body sensation, cough, throat pain, vocal changes, and/or hoarseness (22). To our best knowledge, the relationship between nasal itching and reflux has never been reported. However, it has been reported that reflux was related to rhinitis or sinusitis (4,23). We postulate that the higher proportion of nasal itching in responders might be due to rhinitis triggered by reflux. Similarly, the tickle in the throat may be a special presentation of acid reflux or laryngopharyngeal reflux. In addition to the reflux mechanism, a vagally mediated reflex process in distal esophageal reflux could also cause extraesophageal symptoms (24). The reflex mechanism could explain why most reflux events occurred in the distal esophagus in patients with chronic cough (80%) (25-27). Acidification of the distal esophagus could stimulate acid-sensitive receptors that may interact with pulmonary bronchi and other upper airway structures via a vagally mediated arc (28). This sensory neuronal dysfunction might lead to hypersensitive somatic sensation in the upper airway and present as a constant itch in the throat and nose, causing a neuronal sensitization process (29) and indicating a good response to acid inhibitors. However, the mechanism of neuronal sensitization linking distal reflux and cough remains unclear. Further study needs to figure out the relationship between reflux, nasal itching, and tickle in the throat. In contrast to nasal itching and tickle in the throat, we found that a higher proportion of pharyngeal foreign body sensation existed in non-responders. Pharyngeal foreign body sensation might reflect chronic cough hypersensitivity or refractory chronic cough, indicating poor response to PPI and prokinetics (30,31).
We only compared the DeMeester score and SAP between responders and non-responders. No significant difference was found. According to the 2018 Lyon Consensus, new parameters, AET, and total reflux episodes also come up with definite acid reflux. We did not analyze the two variables in the current study. We began to establish our chronic cough database after 2000, DeMeester score and SAP were recorded in our chronic cough database and were recommended as primary diagnostic variables at that time according to the National Guidelines on the Diagnosis and Management of Cough in China (15). There are several limitations in this study. First, in our clinic, if cough did not improve after four weeks of anti-reflux therapy, anti-reflux empirical treatment would end usually, those patients were defined as non-responders. Not all non-responders were real non-responders since cough might improve after longer treatment occasionally. However, we found that most patients with GERC showed a positive response to anti-reflux treatment within 2 weeks (18). Second, multichannel intraluminal impedance-pH monitoring (MII-pH) was not in use in the early stage of this study. Weakly acidic or weakly alkaline reflux episodes might be missed (32). Third, oesophageal manometry was not used to evaluate impaired oesophageal and gastric motility in this study. It might be valuable to screen out the population that would respond to anti-reflux therapy. In this study, we tried to find clinical features to predict the response of anti-reflux treatment and a lot of variables were compared in the current study. We could not rule out completely the possibility of false positives. Nonetheless, these results could provide a reference for subsequent studies. More prospective studies are needed to further investigate the relationship between these clinical features and the efficacy of anti-reflux therapy for suspected GERC.
Conclusions
Our study showed that over half of suspected GERC patients benefited from anti-reflux therapy. Reflux-related symptoms could not predict the anti-reflux response. Higher proportions of nasal itching, tickle in the throat and a lower proportion of pharyngeal foreign body sensation were found in responders, which might indicate a positive response to anti-reflux treatment in patients with suspected GERC. Further study is needed to identify the predictive value of these clinical features.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Woo-Jung Song, Kian Fan Chung) for the series “Novel Insights Into Chronic Cough” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1046/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1046/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1046/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1046/coif). The series “Novel Insights Into Chronic Cough” was commissioned by the editorial office without any funding or sponsorship. KL serves as an unpaid editorial board member of Journal of Thoracic Disease. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2020150). All patients gave informed consent for their data to be analyzed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morice AH. Epidemiology of cough. Pulm Pharmacol Ther 2002;15:253-9. [Crossref] [PubMed]
- Kahrilas PJ, Altman KW, Chang AB, et al. Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. Chest 2016;150:1341-60. [Crossref] [PubMed]
- Long L, Lai K. Characteristics of Chinese chronic cough patients. Pulm Pharmacol Ther 2019;57:101811. [Crossref] [PubMed]
- Kanemitsu Y, Kurokawa R, Takeda N, et al. Clinical impact of gastroesophageal reflux disease in patients with subacute/chronic cough. Allergol Int 2019;68:478-85. [Crossref] [PubMed]
- Lai K, Huang L, Zhao H, et al. A multicenter survey on the current status of chronic cough and its impact on quality of life in Guangdong, China. J Thorac Dis 2022;14:3624-32. [Crossref] [PubMed]
- Lai K, Zhan W, Li H, et al. The Predicative Clinical Features Associated with Chronic Cough That Has a Single Underlying Cause. J Allergy Clin Immunol Pract 2021;9:426-432.e2. [Crossref] [PubMed]
- Deng HY, Luo W, Zhang M, et al. Initial empirical treatment based on clinical feature of chronic cough. Clin Respir J 2016;10:622-30. [Crossref] [PubMed]
- Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest 2006;129:80S-94S. [Crossref] [PubMed]
- Irwin RS, French CL, Curley FJ, et al. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest 1993;104:1511-7. [Crossref] [PubMed]
- Novitsky YW, Zawacki JK, Irwin RS, et al. Chronic cough due to gastroesophageal reflux disease: efficacy of antireflux surgery. Surg Endosc 2002;16:567-71. [Crossref] [PubMed]
- Ours TM, Kavuru MS, Schilz RJ, et al. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol 1999;94:3131-8. [Crossref] [PubMed]
- Lai KF, Chen RC, Liu CL, et al. Etiology and a diagnostic protocol for patients with chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:96-9. [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992;87:1102-11. [PubMed]
- Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association (CMA). Guidelines for diagnosis and management of cough (2015). Zhonghua Jie He He Hu Xi Za Zhi 2016;39:323-54.
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. [Crossref] [PubMed]
- Zhu LX, Ma HM, Lai KF, et al. Clinical analysis of gastroesophageal reflux induced cough. Zhonghua Nei Ke Za Zhi 2003;42:461-5. [PubMed]
- Liu CL, Lai KF, Chen RC, et al. The clinical features and the diagnosis of gastro-esophageal reflux induced cough. Zhonghua Nei Ke Za Zhi 2005;44:438-41. [PubMed]
- Hersh MJ, Sayuk GS, Gyawali CP. Long-term therapeutic outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol 2010;44:254-60. [Crossref] [PubMed]
- Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014;145:1264-70. [Crossref] [PubMed]
- Vaezi MF, Hicks DM, Abelson TI, et al. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 2003;1:333-44. [Crossref] [PubMed]
- Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005;294:1534-40. [Crossref] [PubMed]
- Feng MC, Tsai YG, Chang YH, et al. Allergic rhinitis as a key factor for the development of gastroesophageal reflux disease in children. J Microbiol Immunol Infect 2021;54:1167-74. [Crossref] [PubMed]
- Wu J, Ma Y, Chen Y. GERD-related chronic cough: Possible mechanism, diagnosis and treatment. Front Physiol 2022;13:1005404. [Crossref] [PubMed]
- Decalmer S, Stovold R, Houghton LA, et al. Chronic cough: relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest 2012;142:958-64. [Crossref] [PubMed]
- Chen Z, Sun L, Chen H, et al. Dorsal Vagal Complex Modulates Neurogenic Airway Inflammation in a Guinea Pig Model With Esophageal Perfusion of HCl. Front Physiol 2018;9:536. [Crossref] [PubMed]
- Liu C, Chen R, Luo W, et al. Neurogenic airway inflammation induced by repeated intra-esophageal instillation of HCl in guinea pigs. Inflammation 2013;36:493-500. [Crossref] [PubMed]
- Hom C, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Gastroenterol Clin North Am 2013;42:71-91. [Crossref] [PubMed]
- Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010;139:754-62. [Crossref] [PubMed]
- Bredenoord AJ. Impedance-pH monitoring: new standard for measuring gastro-oesophageal reflux. Neurogastroenterol Motil 2008;20:434-9. [Crossref] [PubMed]
- Li N, Chen Q, Wen S, et al. Diagnostic accuracy of multichannel intraluminal impedance-pH monitoring for gastroesophageal reflux-induced chronic cough. Chron Respir Dis 2021;18:14799731211006682. [Crossref] [PubMed]
- Wang S, Wen S, Bai X, et al. Diagnostic value of reflux episodes in gastroesophageal reflux-induced chronic cough: a novel predictive indicator. Ther Adv Chronic Dis 2022;13:20406223221117455. [Crossref] [PubMed]


