
Efficacy and safety analysis of anlotinib combined with PD-1 inhibitors in advanced non-small cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• This study indicated that anlotinib combined with programmed cell death 1 (PD-1) inhibitors provides an effective and safe treatment strategy for patients with advanced lung cancer.
What is known and what is new?
• The use of PD-1 inhibitors in the treatment of lung cancer has been widely reported.
• We examined the use of anlotinib combined with PD-1 inhibitors in the treatment of advanced non-small cell lung cancer, which has rarely been reported in previous studies.
What is the implication, and what should change now?
• We found that anlotinib combined with PD-1 inhibitor therapy is effective and safe in the treatment of non-small cell lung cancer. In the future, the further research needs to be conducted on the overall survival of the patients who receive this treatment.
Introduction
Due to the absence of effective screening programs and the late onset of symptoms, lung cancer is mostly diagnosed at an advanced stage, and has a 5-year survival rate of only 5% (1). As a treatment for non-small cell lung cancer (NSCLC), classic chemotherapy has only shown overall response rates of 6.7–10.8% (2) and 5-year survival rates of 7–14% (3).
Immune checkpoint inhibitors (ICIs) can restore active T-cell infiltration, stimulate cancer-specific immune responses, and improve the long-term survival and durable response (4). In addition to the traditional gold standards overall survival (OS) and objective response rate (ORR), the unique evaluation standards for ICIs such as treatment-free time survival (5) and durable responses (6), which are distinctively clinical benefits of ICIs. ICIs rechallenge might be an effective therapy for patients who discontinue treatment due to immune-related adverse events (AEs) (7). ICIs have been shown to prolong the survival time of advanced lung cancer patients (8). However, only about 20% of NSCLC patients benefit from immune monotherapy (9,10). Thus, it is necessary to explore combined therapy strategies to improve the efficacy of immunotherapy.
There is increasing evidence that antiangiogenic agents regulate T cells, which modulate the systemic effects of immune cell function (11) and improve tumor vascular perfusion and oxygenation (12). Combining immunotherapy and antiangiogenic agents may synergistically increase treatment efficacy. Anlotinib is a small-molecule multi-targeted antiangiogenic agent that has an inhibitory action not only on tumor cells but also on angiogenesis (13). Anlotinib could stimulate the infiltration of the innate immune cells (14). The therapy of anlotinib combined with programmed cell death 1 (PD-1) inhibitors is efficacy, durability, and safety (15). This combination of immunotherapy and anlotinib may provide an effective treatment for advanced lung cancer patients who cannot tolerate chemotherapy or have non-treatable gene mutations. However, evidence on the efficacy and safety of anlotinib combined with PD-1 inhibitors in the treatment of advanced NSCLC is limited. The diversity and flexibility of real-world research make it powerful evidence for responding to practical clinical difficulties. Thus, we retrospectively explored the efficacy and safety of the combination of anlotinib and PD-1 inhibitors in advanced NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-289/rc).
Methods
Patients and treatment
This was a retrospective, longitudinal study. The medical records of advanced NSCLC patients who received anlotinib and PD-1 inhibitors from May 2020 to November 2022 at the Shanghai Chest Hospital, China were retrospectively reviewed. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have pathologically confirmed stage IIIB–IV NSCLC according to the 8th edition of the American Joint Committee on Cancer staging manual; and (II) have received anlotinib (10 mg/12 mg) and PD-1 inhibitors (including pembrolizumab, nivolumab, tislelizumab, sintilimab, and camrelizumab). Patients without complete medical records or follow-up information affected the evaluation of efficacy and safety were excluded from the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the Shanghai Chest Hospital approved the study (No. IS22010), and written consent was obtained from all patients.
Data collection and follow-up
The clinical data, including the age, gender, pathological type, clinical stage, smoking history, treatment line, and metastatic site. Therapeutic and prognostic information were retrospectively collected. Clinical response was assessed according to the Response Evaluation Criteria in Solid Tumor (version 1.1) by computed tomography (CT) scans every 6 weeks (±7 d), and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was defined as achieving a CR or PR. The disease control rate (DCR) was defined as achieving a CR, PR, or SD. The disease status and survival status data were acquired from the follow-up records, and the progression-free survival (PFS) and overall survival (OS) of the patients were then calculated. PFS was defined as the time from which the oral administration of anlotinib was started to PD or to the last follow-up. The adverse events (AEs) were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Statistical analysis
The Chi-square test and Fisher’s exact test were used to compare the differences between the subgroups in terms of the clinical characteristics and efficacy. PFS was analyzed by Kaplan-Meier method. Hazard ratio (HR) was estimated with the use of a Cox proportional-hazards model. SPSS 25.0 statistical software (IBM, USA) was used to perform the statistical analysis and generate the figures. A 2-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 48 initially screened patients, 6 were excluded due to incomplete medical records or follow-up information. Thus, a total of 42 patients were included in the study. The patients had a median age of 63 years (range, 47–81 years). Of the 42 patients, 33 (78.6%) were male patients, 25 (59.5%) were ever smokers, and 27 (64.3%) patients were diagnosed with adenocarcinomas. Most of the patients (35, 83.3%) were confirmed to have stage IV NSCLC at the time of diagnosis. Among the patients, 10 (23.8%) had the epidermal growth factor receptor (EGFR) mutation, and 3 (7.1%) had brain metastases, and 6 (14.3%) had liver metastases. The number of patients with programmed cell death-ligand 1 (PD-L1) <1%, PD-L1 1–49%, PD-L1 ≥50%, or PD-L1 unknown was 10 (23.8%), 12 (28.6%), 2 (4.8%), and 18 (42.9%), respectively. The baseline characteristics among the patients with the 4 PD-L1 expression subtypes did not differ significantly. In this study,34 patients had treated chemotherapy, 10 patients had treated EGFR-TKI, and 11 patients had treated ICIs. At the data cut-off date, the median follow-up duration was 13.973 months (range, 1.282–25.085 months) (Table 1).
Table 1
| Characteristics | PD-L1 <1% (n=10), n (%) |
PD-L1 1–49% (n=12), n (%) |
PD-L1 ≥50% (n=2), n (%) |
PD-L1 unknown (n=18), n (%) | P value |
|---|---|---|---|---|---|
| Age (years) | 0.316 | ||||
| <65 | 6 (60.0) | 9 (75.0) | 0 (0.0) | 11 (61.1) | |
| ≥65 | 4 (40.0) | 3 (25.0) | 2 (100.0) | 7 (38.9) | |
| Sex | 0.519 | ||||
| Male | 7 (70.0) | 11 (91.7) | 2 (100.0) | 13 (72.2) | |
| Female | 3 (30.0) | 1 (8.3) | 0 (0.0) | 5 (27.8) | |
| Smoking history | 0.282 | ||||
| No | 4 (40.0) | 3 (25.0) | 0 (0.0) | 10 (55.6) | |
| Ever | 6 (60.0) | 9 (75.0) | 2 (100.0) | 8 (44.4) | |
| Histology | 0.069 | ||||
| Adenocarcinoma | 5 (50.0) | 7 (58.3) | 0 (0.0) | 15 (83.3) | |
| Squamous | 4 (40.0) | 4 (33.3) | 2 (100.0) | 2 (11.1) | |
| Sarcoma | 1 (10.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | |
| NSCLC | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | |
| Stage | 0.185 | ||||
| IIIB/IIIC | 3 (30.0) | 0 (0.0) | 0 (0.0) | 4 (22.2) | |
| IV | 7 (70.0) | 12 (100.0) | 2 (100.0) | 14 (77.8) | |
| Gene status | |||||
| EGFR | 2 (20.0) | 1 (8.3) | 0 (0.0) | 7 (38.9) | 0.569 |
| TP53 | 1 (10.0) | 2 (16.7) | 0 (0.0) | 2 (11.1) | |
| Other | 7 (70.0) | 9 (75.0) | 2 (100.0) | 9 (50.0) | |
| Brain metastasis | 0.408 | ||||
| No | 10 (100.0) | 12 (100.0) | 2 (100.0) | 15 (83.3) | |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (16.7) | |
| Liver metastasis | 0.474 | ||||
| No | 9 (90.0) | 11 (91.7) | 1 (50.0) | 15 (83.3) | |
| Yes | 1 (10.0) | 1 (8.3) | 1 (50.0) | 3 (16.7) | |
| Anlotinib dose | 0.732 | ||||
| 10 mg | 5 (50.0) | 7 (58.3) | 2 (100.0) | 9 (50.0) | |
| 12 mg | 5 (50.0) | 5 (41.7) | 0 (0.0) | 9 (50.0) |
PD-L1, programmed cell death-ligand 1; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor.
Efficacy evaluation
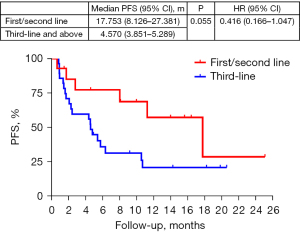
The overall median PFS (mPFS) (data cut-off date: 25 December 2022) was 5.721 months [95% confidence interval (CI): 1.365–10.076 months]. The between subgroup comparisons revealed significant differences in the PFS and ORRs based on sex. Specifically, the male patients had a longer mPFS (10.554 months; 95% CI: 2.741–18.366 months) than the female patients (4.340 months; 95% CI: 0.723–7.956 months, P=0.010). The ORRs and DCRs of male patients and female patients were 36.4% vs. 0.0% (P=0.041) and 78.8% vs. 55.6% (P=0.209). The mPFS of the patients who received anlotinib plus PD-1 inhibitors as a first-, second-, and third-line and above therapy were 17.753, 11.244, and 4.570 months, respectively (P=0.103). The ORRs of the patients who received anlotinib plus PD-1 inhibitors as a first-, second-, and third-line and above therapy were statistically significant by 87.5% vs. 16.7% vs. 14.3% (P<0.001). The DCRs of the patients who received anlotinib plus PD-1 inhibitors as a first-, second-, and third-line and above therapy were 100.0%, 83.3%, and 64.3% (P=0.096). Further sub-combined analysis of mPFS for first/second line therapy and third line and above therapy were 17.753 and 4.570 months (P=0.055, HR 0.416, 95% CI: 0.166–1.047, Figure 1). The mPFS of the stage III patients was not reached, the ORR was 28.6%, and the DCR was 100.0%. The mPFS of the stage IV patients was 5.392 months, the ORR was 28.6%, and the DCR was 68.6%. In terms of the different pathological types, the sarcoma patients had a significantly higher ORR (100.0% vs. 33.3% vs. 18.5%, P=0.025) and DCR (100.0% vs. 83.8% vs. 66.7%, P=0.692) than the squamous and adenocarcinoma patients, respectively.
The patients with the tumor protein 53 (TP53) mutation had a significantly higher DCR (100.0% vs. 40.0% vs. 81.5%, P=0.020) than those with the EGFR and other mutations, respectively. Similarly, the patients with the TP53 mutation had a better PFS (10.685 vs. 2.038 vs. 7.989 months, P=0.144) and ORR (40.0% vs. 10.0% vs. 33.3%, P=0.406) than those with the EGFR and other mutations, respectively. There was no statistically significant difference in the treatment efficacy among the patients with different expressions of PD-L1 (PD-L1 negative, 1–49%, and ≥50%), and their mPFS were 7.989, 2.762, 1.414, and 5.392, months, respectively (P=0.849), their ORRs were 40.0%, 25.0%, 50.0%, and 22.2%, respectively (P=0.557), and their DCRs were 80.0%, 75.0%, 50.0%, and 72.2%, respectively (P=0.817, Table 2).
Table 2
| Characteristics | N | PFS | ORR | DCR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median (95% CI) (m) | P value | n (%) | P value | n (%) | P value | ||||
| Age (years) | 0.468 | 1.000 | 0.720 | ||||||
| <65 | 26 | 5.721 (3.212–8.230) | 7 (26.9) | 20 (76.9) | |||||
| ≥65 | 16 | NR | 5 (31.3) | 11 (68.8) | |||||
| Sex | 0.010 | 0.041 | 0.209 | ||||||
| Male | 33 | 10.553 (2.741–18.366) | 12 (36.4) | 26 (78.8) | |||||
| Female | 9 | 4.340 (0.723–7.956) | 0 (0) | 5 (55.6) | |||||
| Smoking history | 0.266 | 0.300 | 0.733 | ||||||
| No | 17 | 4.570 (4.127–5.012) | 3 (17.6) | 12 (70.6) | |||||
| Ever | 25 | 7.989 (0.243–15.735) | 9 (36.0) | 19 (76.0) | |||||
| Histology | 0.173 | 0.025 | 0.692 | ||||||
| Adenocarcinoma | 27 | 4.570 (1.476–7.664) | 5 (18.5) | 18 (66.7) | |||||
| Squamous | 12 | 7.989 (3.370–12.608) | 4 (33.3) | 10 (83.8) | |||||
| Sarcoma | 2 | NR | 2 (100.0) | 2 (100.0) | |||||
| NSCLC | 1 | NR | 1 (100.0) | 1 (100.0) | |||||
| Stage | 0.066 | 1.000 | 0.161 | ||||||
| IIIB/IIIC | 7 | NR | 2 (28.6) | 7 (100.0) | |||||
| IV | 35 | 5.392 (3.437–7.347) | 10 (28.6) | 24 (68.6) | |||||
| Gene status | 0.144 | 0.406 | 0.020 | ||||||
| EGFR | 10 | 2.038 (0.969–3.108) | 1 (10.0) | 4 (40.0) | |||||
| TP53 | 5 | 10.685 (NR–NR) | 2 (40.0) | 5 (100.0) | |||||
| Other | 27 | 7.989 (0.756–15.222) | 9 (33.3) | 22 (81.5) | |||||
| PD-L1 expression | 0.849 | 0.557 | 0.817 | ||||||
| <1% | 10 | 7.989 (0.000–20.092) | 4 (40.0) | 8 (80.0) | |||||
| 1–49% | 12 | 2.762 (0.000–8.398) | 3 (25.0) | 9 (75.0) | |||||
| ≥50% | 2 | 1.414 (NR–NR) | 1 (50.0) | 1 (50.0) | |||||
| Unknown | 18 | 5.392 (2.703–8.080) | 4 (22.2) | 13 (72.2) | |||||
| Treatment line | 0.103 | <0.001 | 0.096 | ||||||
| First-line | 8 | 17.753 (3.668–31.839) | 7 (87.5) | 8 (100.0) | |||||
| Second-line | 6 | 11.244 (NR–NR) | 1 (16.7) | 5 (83.3) | |||||
| Third-line and above | 28 | 4.570 (3.851–5.289) | 4 (14.3) | 18 (64.3) | |||||
| Brain metastasis | 0.074 | 0.545 | 0.163 | ||||||
| No | 39 | 7.989 (0.909–15.069) | 12 (30.8) | 30 (76.9) | |||||
| Yes | 3 | 2.038 (1.302–2.775) | 0 (0.0) | 1 (33.3) | |||||
| Liver metastasis | 0.379 | 0.655 | 0.644 | ||||||
| No | 36 | 6.312 (0.000–12.902) | 11 (30.6) | 27 (75.0) | |||||
| Yes | 6 | 4.340 (0.000–9.114) | 1 (16.7) | 4 (66.7) | |||||
| Anlotinib dose | 0.821 | 0.495 | 1.000 | ||||||
| 10 mg | 23 | 5.721 (3.085–8.356) | 8 (34.8) | 17 (73.9) | |||||
| 12 mg | 19 | 7.989 (0.00–16.570) | 4 (21.1) | 14 (73.7) | |||||
PD-1, programmed cell death 1; NSCLC, non-small cell lung cancer; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; CI, confidence interval; m, months; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death-ligand 1; NR, not reached.
Safety
All-grade AEs occurred in 22 patients (52.38%) and mainly included fatigue (45.24%), anorexia (40.48%), hypertension (30.95%), pneumonia (7.14%), and hemoptysis (7.14%). The grades 3 AEs were hypertension (7.14%), pneumonia (2.38%), and mucositis oral (2.38%). A total of 3 patients discontinued treatment due to anemia, mucositis oral, and pneumonia respectively (Table 3).
Table 3
| AEs | Any, n (%) | Grade 3, n (%) |
|---|---|---|
| Total | 22 (52.38) | 5 (11.90) |
| Fatigue | 19 (45.24) | 0 (0) |
| Anorexia | 17 (40.48) | 0 (0) |
| Hypertension | 13 (30.95) | 3 (7.14) |
| Pneumonia | 3 (7.14) | 1 (2.38) |
| Hemoptysis | 3 (7.14) | 0 (0) |
| Creatinine increased | 2 (4.76) | 0 (0) |
| Mucositis oral | 1 (2.38) | 1 (2.38) |
| Palmar-plantar erythrodysesthesia syndrome | 1 (2.38) | 0 (0) |
| Proteinuria | 1 (2.38) | 0 (0) |
| Hypothyroidism | 1 (2.38) | 0 (0) |
| Anemia | 1 (2.38) | 0 (0) |
AE, adverse event.
Discussion
Anlotinib is an oral multi-targeted tyrosine kinase inhibitor (TKI) that selectively inhibits the vascular endothelial growth factors 1–3, fibroblast growth factors 1–4, platelet-derived growth factor, C-proto-oncogenic receptor tyrosine kinase, and proto-oncogene rearrangement during transfection (RET). It has the dual effects of regulating tumor angiogenesis and repressing tumor cell growth. Advanced NSCLC patients who received anlotinib as a third-line or above therapy have been reported to have a mPFS of 5.4 months, a mOS of 9.6 months, an ORR of 9.2%, and a DCR of 81.0% (16). Anlotinib could improve PFS in NSCLC patients with liver metastases (17). Patients with brain metastases treated with anlotinib have been reported to have a mPFS of 4.17 months and a mOS of 8.57 months. Additionally, the following patients have been reported to have a significantly longer time to brain progression: those aged >60 years, those with EGFR mutations, non-smokers, those with an Eastern Cooperative Oncology Group performance status of 1, those with IV stage, and those who have previously received targeted TKI therapy, or surgery (18).
PD-L1 expression is not the only criterion to evaluate the efficacy of anti-PD-1 therapies, others such as tumor-infiltrating lymphocytes, mutational burden, immune gene signatures, and multiplex immunohistochemistry are effective predictive biomarker (19). Neoadjuvant nivolumab plus chemotherapy resulted in significantly longer disease-free survival than chemotherapy alone (20). The immunostimulatory properties of the treatment appear to be positively correlated with the dose of anlotinib, which differs from other antiangiogenic agents (21,22). Anlotinib could stimulate the infiltration of the innate immune cells (e.g., the natural-killer cells and antigen-presenting cells), and thus convert the tumor immune microenvironment from an immune-suppressive to immune-supportive phenotype. Anlotinib has been shown to upregulate interferon (IFN)-γ expression in CD4+ T cells and to significantly reduce the percentages of M2-like tumor-associated macrophages (14).
Previous studies have confirmed the efficacy of anlotinib combined with PD-1 inhibitors in the treatment of advanced NSCLC patients. In one study, advanced NSCLC patients without EGFR/anaplastic lymphoma kinase (ALK)/tyrosine kinase receptor c-ros-oncogene 1 (ROS1) mutations received sintilimab and anlotinib as a first-line therapy and achieved a DCR of 100%, a mPFS of 15 months, and a 12-month PFS rate of 71.4% (15). Compared to mOS of 9.2–13.8 months in ICI monotherapy (23), the patients who received the combination treatment as a second-line and above treatment had a mPFS of 11.4 months, a mOS of 27.0 months, an ORR of 40.0%, and a DCR of 82.5% (24). The most common AEs were fatigue (45.5%), anorexia (40.9%), and hypertension (45.5%). The ≥3 grade AEs were hypertension (9.1%), mouth ulceration (9.1%), rash (9.1%), pneumonitis (4.6%), and diarrhea (4.6%) (25).
In this study, we found that the PFS of the patients treated with anlotinib combined with PD-1 inhibitors as a first-, second-, and third-line and above therapy were 17.753, 11.244, and 4.57 months, respectively, while the DCRs were 100%, 83.3%, and 64.3%, respectively. Further sub-combined analysis of mPFS for first/second line therapy and third line and above therapy were 17.753 and 4.570 months (P=0.055, HR 0.416, 95% CI: 0.166–1.047). Numerical values show a difference and P values are close to statistically significant. Thus, this treatment appears to have a better effect as an early line treatment and especially used before the third line. The sarcoma and TP53 mutation patients achieved a DCR of 100%. The male patients benefited more than the female patients. Anlotinib combined with PD-1 inhibitors is a useful treatment for patients after an EGFR-TKI treatment. There was no significant difference in clinical efficacy between patients with different PD-1 expression levels and different PD-1 inhibitors. The 10 mg and 12 mg doses of anlotinib had similar efficacy. All-grade AEs occurred in 52.38% of the patients.
The grade 3 AEs were hypertension (7.14%), pneumonia (2.38%), and mucositis oral (2.38%). Further, the AEs occurred in the patients who received 10 mg of anlotinib were less than those who received 12 mg of anlotinib. A total of 3 patients discontinued treatment due to anemia, mucositis oral, and pneumonia, respectively.
This study has a few limitations. First, the relatively small sample size may affect the results, descriptive data for efficacy and safety profile are needed to be confirmed in future large-scale studies. Second, this is an observational study without setting a matched control group for PD-1 monotherapy or PD-1 inhibitors combined with chemotherapy, our results in line with some of previous studies and can provide a degree of real-world understanding of anlotinib combined with PD-1 inhibitors in advanced NSCLC patients. Despite these limitations, our study enriches the clinical evidence for the efficacy and safety of anlotinib combined with PD-1 inhibitors in patients with advanced NSCLC. In the future, the efficacy and safety assessment of anlotinib combined with PD-1 inhibitors should be explored in prospective clinical trials with larger sample sizes.
Conclusions
This real-world observational study showed that anlotinib combined with PD-1 inhibitors has good efficacy and a well-tolerated safety profile in advanced NSCLC patients. The combination therapy provides a new strategy for patients with advanced lung cancer.
Acknowledgments
Funding: This study was supported by the Beijing Medical and Health Foundation (No. YWJKJJHKYJJ-F2200E), the Shanghai Science and Technology Committee (Nos. 22Y31920400, and 22Y31920404), the Clinical Research Plan of SHDC (No. SHDC2020CR1052B), and the National Natural Science Foundation of China (No. 82274595).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-289/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-289/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-289/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the Shanghai Chest Hospital approved the study (No. IS22010), and all the patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022;72:409-36. [Crossref] [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 2023;41:1200-12. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol 2019;5:1411-20. [Crossref] [PubMed]
- Regan MM, Werner L, Rao S, et al. Treatment-Free Survival: A Novel Outcome Measure of the Effects of Immune Checkpoint Inhibition-A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2019;37:3350-8. [Crossref] [PubMed]
- Michielin O, Lalani AK, Robert C, et al. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J Immunother Cancer 2022;10:e003024. [Crossref] [PubMed]
- Yang J, Zeng R, Zhou J, et al. Efficacy, prognosis and safety analysis of anti-PD-1/PD-L1 inhibitor rechallenge in advanced lung cancer patients: a cohort study. Transl Lung Cancer Res 2022;11:1038-50. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1‒Positive, Advanced Non‒Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol 2020;38:1580-90. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Manegold C, Dingemans AC, Gray JE, et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J Thorac Oncol 2017;12:194-207. [Crossref] [PubMed]
- Farsaci B, Donahue RN, Coplin MA, et al. Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol Res 2014;2:1090-102. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Yang Y, Li L, Jiang Z, et al. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother 2020;69:2523-32. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Shen Y, Lu J, Hu F, et al. Effect and outcomes analysis of anlotinib in non-small cell lung cancer patients with liver metastasis: results from the ALTER 0303 phase 3 randomized clinical trial. J Cancer Res Clin Oncol 2023;149:1417-24. [Crossref] [PubMed]
- Jiang S, Liang H, Liu Z, et al. The Impact of Anlotinib on Brain Metastases of Non-Small Cell Lung Cancer: Post Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303). Oncologist 2020;25:e870-4. [Crossref] [PubMed]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-51. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012;109:17561-6. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Yuan S, Peng L, Liu Y, et al. Low-dose anlotinib confers improved survival in combination with immune checkpoint inhibitor in advanced non-small cell lung cancer patients. Cancer Immunol Immunother 2023;72:437-48. [Crossref] [PubMed]
- Zhai C, Zhang X, Ren L, et al. The Efficacy and Safety of Anlotinib Combined With PD-1 Antibody for Third-Line or Further-Line Treatment of Patients With Advanced Non-Small-Cell Lung Cancer. Front Oncol 2020;10:619010. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


