
Efficacy and safety of Lianhua Qingwen capsules combined with standard of care in the treatment of adult patients with mild to moderate COVID-19 (FLOSAN): protocol for a randomized, double-blind, international multicenter clinical trial
Introduction
Coronavirus disease 2019 (COVID-19), caused by a novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)], has become a public health emergency causing international concern since its outbreak (1). More than 460 million cases have been confirmed and more than 6 million deaths have occurred as of March 2022 (2). As the number of infections increases and the epidemic continues, SARS-CoV-2 continues to evolve and mutate resulting in the emergence of new variants (3,4), which increases the uncertainty and complexity of the global spread of COVID-19. Current evidence shows that more than 80% of COVID-19 cases are mild or moderate (5,6). Therefore, initiating treatment in the early phase of infection to reduce the risk of hospitalization and minimize transmission is a key component of current clinical care.
Lianhua Qingwen (LHQW) is a new Chinese patent medicine for the treatment of cold and flu approved by the China’s National Medical Products Administration (NMPA). This formula is composed of 13 Chinese herbs, including Forsythiae Fructus, Lonicerae Japonicae Flos, Ephedrae Herba (processed with honey), Armeniacae Semen Amarum (stir-baked), Gypsum Fibrosum, Isatidis Radix, Dryopteridis Crassirhizomatis Rhizoma, Houttuyniae Herba, Pogostemonis Herba, Rhei Radix et Rhizoma, Rhodiolae Crenulatae Radix et Rhizoma, l-Menthol, Glycyrrhizae Radix et Rhizoma. Basic experimental research showed that LHQW could inhibit the cytopathic effect of SARS-CoV-2-infected VeroE6 cells, significantly reduce the number of cell virus particles, and inhibit the overexpression of inflammatory (7). Additionally, LHQW effectively inhibited weight loss and improved lung inflammatory injury in SARS-CoV-2-infected mice (7). Clinical studies showed that in addition to the standard of care (SOC), LHQW improved the clinical symptoms of COVID-19 (including fever, cough, expectoration, and shortness of breath) and significantly shorted the duration of fever (8-10). A real-world study on the administration of LHQW to prevent COVID-19 in quarantined people confirmed that the positivity rate of SARS-CoV-2 nucleic acid in nasal and throat swabs of participants was significantly lower in the LHQW group than that in the control group (11). In summary, LHQW played an active role in the prevention and control of COVID-19 during the outbreak of the epidemic in China. Hence, in addition to the originally approved indication, LHQW is approved for the new indication of the treatment of mild to moderate COVID-19 by the China’s NMPA in 2020. It is also included in the National Diagnosis and Treatment Protocol for COVID-19 (Editions 4 through 9) in China.
However, there are no reports on whether LHQW exhibits the same clinical efficacy in different countries and ethnic groups. More importantly, in the context of a large number of infected people, heavy social and economic burden, and overstretched healthcare system, there is also a serious lack of pharmacoeconomic evaluation of COVID-19 therapeutic drugs. Therefore, to evaluate the clinical efficacy, safety, and economy of LHQW in the treatment of COVID-19 and to provide clinical evidence for international treatment regimens for COVID-19, an international clinical study is designed. The name of the study is FLOSAN. “Flos” means flower in Latin and is also the Latin abbreviation of Lonicerae Japonicae Flos, which is one of the components of LHQW; “San” is short for the Latin word “SANUS” which means health and is also a root associated with health and hygiene in English. Therefore, FLOSAN, the name of this study, suggests the role of LHQW in protecting health. We present this article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-281/rc).
Methods
Study design
This is a randomized, double-blind, placebo-controlled, international multicenter trial to evaluate the clinical efficacy, safety, and economy of LHQW in the treatment of adult patients with mild to moderate COVID-19. Subjects will be recruited from ten hospitals designated for COVID-19 treatment with sufficient experience in RCTs. Six of the hospitals are in China, while the remaining four are in Cambodia, Thailand, Vietnam and Singapore. Eligible patients who meet the inclusion criteria and do not meet the exclusion criteria are assigned randomly to the LHQW or placebo group and are administered a 2-week treatment. The study flowchart is shown in Figure 1. Patients are assessed on days 0, 3, 7, 10, and 14. If necessary, the investigator could add follow-up visits after the end of treatment due to persistent adverse events or clinically significant laboratory or vital sign abnormalities, and follow-up by the investigator is performed on a case-by-case basis by telephone. The specific assessments at each visit are detailed in Table 1.
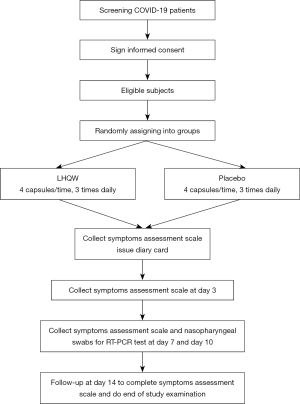
Table 1
| Evaluated items | Screening Stage | Treatment stage | End of treatment | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (−4 to 0 days) |
Visit 2 (3±1 days) |
Visit 3 (7±1 days) |
Visit 4 (10±1 days) |
Visit 5 (14±1 days) |
Visit 6 (30±3 days) |
||||
| Signing informed consent | X | ||||||||
| Rapid antigen test | X | ||||||||
| Nucleic acid test from nasopharyngeal swab | X | X | X | X | |||||
| Gene sequence analysis (at conditional centers) | X | ||||||||
| Subjects are screened according to entry criteria | X | ||||||||
| Demographic statistics | X | ||||||||
| History of illness | X | ||||||||
| Vital signs | X | X | X | X | X | ||||
| COVID-19 symptom scale (diary card)a | X | X | X | X | X | ||||
| EQ-5D-5Lb | X | X | X | X | X | X | |||
| Cost scalec | X | X | X | X | X | X | |||
| Combination of medication | X | X | X | X | X | ||||
| Electrocardiogram | X | X | |||||||
| Blood cell analysis | X | X | |||||||
| Urine chemical analysis | X | X | |||||||
| Liver and kidney function | X | X | |||||||
| Cardiac enzymes | X | X | |||||||
| Inflammation indicatorsd,e | X | X | |||||||
| Chest CT/chest X-ray (centers with access) | X | X | |||||||
| Randomization | X | ||||||||
| Experimental drug distribution | X | ||||||||
| Recycling the experimental drug | X | ||||||||
| Adverse events collection | X | X | X | X | |||||
| Outcome | X | X | X | X | |||||
a, the COVID-19 symptom scale (see Figure 2 for details) evaluation requires input twice daily, such as in the morning and evening, and assessment on days 0, 3, 7, 10, and 14; b, EQ-5D-5L is required to be collected on days 0, 3, 7, 10, and 14; c, cost scale is required to be collected on days 0, 3 [1–3], 7 [4–7], 10 [8–10], and 14 [11–14]; d, inflammation indicators include white blood cell counts, lymphocyte counts, C-reactive protein, D-dimer; e, inflammation indicators at the sites in Vietnam, serum samples are additionally collected for detection of interleukin IL-6 and D-dimer on days 0, 2, 4, 6, 8, 10, 12, and 14 during the visit period, with a total of eight times. COVID-19, coronavirus disease 2019.
Study procedure
The diagnosis of COVID-19 follows the disease definition by the World Health Organization (WHO) (12). The inclusion and exclusion criteria of patients are as follows.
Inclusion criteria
- Aged between 18 and 70 years old, male or female.
- Patients who have positive results on the SARS-CoV-2 rapid antigen test or reverse transcription-polymerase chain reaction (RT-PCR) meet the criteria for mild to moderate COVID-19.
WHO diagnostic criteria for mild to moderate COVID-19 (12) (mainly in countries or regions outside China): mild is defined as symptomatic patients meeting the case definition for COVID-19 without evidence of viral pneumonia or hypoxia; moderate is defined with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) but no signs of severe pneumonia, including SpO2 ≥90% on ambient air.
Chinese diagnostic criteria for mild to moderate COVID-19 (13): mild means the clinical symptoms are mild, and there is no sign of pneumonia on imaging; moderate means showing fever and respiratory symptoms with imaging findings of pneumonia.
- No longer than 4 days between the onset of symptoms and screening. [The onset of symptoms is defined as the occurrence of at least one of the following symptoms: cough, sore throat, stuffy or runny nose, shortness of breath (difficulty breathing), muscle or body aches, low energy or tiredness, headache, chills or shivering, feeling hot or feverish, vomiting, nausea, diarrhea, new loss of smell and new loss of taste.]
- At least three of the nine major symptoms appeared within 12 h before screening. [The nine major symptoms are stuffy or runny nose, cough, sore throat, shortness of breath (difficulty breathing), muscle or body aches, low energy or tiredness, chills or shivering, headache, and feeling hot or feverish.]
- Patients who understand and agree with the requirements of the protocol and provide signed written informed consent.
Exclusion criteria
Patients who suffer from severe or critical COVID-19.
- Patients with any type of serious chronic systemic diseases that may affect the efficacy evaluation and disease regression (e.g., poorly controlled diabetes and/or blood pressure, severe obesity, tumors, severe cardiovascular diseases, chronic lung diseases, chronic kidney diseases, chronic liver diseases, diseases that seriously affect the immune system, etc.)
- Patients with known other infections comorbidities.
- Patients who have a history of alcohol or drug abuse (excluding cannabis) within 1 year prior to enrollment.
- Patients enrolled in other clinical studies within 1 month prior to screening. If the half-life of the investigational product is long, the study interval should exceed 5 half-lives.
- Patients with a history of known or possible allergy or hypersensitivity to LHQW and its excipients.
- Pregnant women, lactation or postpartum women with 2 weeks.
- Patients who are considered unsuitable for participation in this study by the investigator.
Discontinuation and withdrawal criteria
The reasons for the subject’s withdrawal of informed consent or discontinuation of the study treatment will be fully recorded. Patients will be removed from the study treatment for any of the following reasons:
- Subjects who have an acute exacerbation leading to the progression to severe disease during the trial. Appropriate treatment should be given according to local diagnosis and treatment guidelines, and follow-up should be performed for 14 days. Clinical outcomes/conditions should be documented in the participant’s medical record.
- Subjects who develop allergic reactions or serious adverse events. The treatment should be discontinued according to the judgment of the investigator.
- Subjects who experience serious complications or specific physiological changes during the trial that are unsuitable to continue the study.
- Subjects with poor medication adherence (<80% of dose) or who no longer receive dosing and testing are considered unsuitable to continue in the trial. The reasons for their withdrawal should be recorded in detail.
- For whatever reason, the patient is unwilling or unable to continue the trial and requests withdrawal from the trial.
Termination criteria
- Serious safety issues occur during the trial.
- Significant errors in the trial protocol are identified during the trial, making it difficult to evaluate the effects of the drug.
- Important deviations occur in implementation, making it difficult to evaluate drug effects.
- There is no need to continue the study due to unacceptable efficacy.
- Request from the sponsor (due to funding or regulation issues).
Interventions
Treatment regimens
Subjects satisfying all criteria will be divided (1:1) randomly into two groups as follows:
- LHQW group: LHQW 1.4 g three times daily in addition to SOC for 14 days.
- placebo group: placebo 1.4 g three times daily in addition to SOC for 14 days.
LHQW and placebo are manufactured by Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijiazhuang, Hebei Province, China), following the People’s Republic of China’s Pharmacopoeia. LHQW and placebo are the same in color, odor, and appearance. These two products will be provided by the sponsor in a blinded manner. All the remaining drugs will be recycled and destroyed after the completion of the trial.
SOC refers to the Living Guidance for Clinical Management of COVID-19 (WHO, 2021.01). Symptomatic treatment with antipyretic analgesics, adequate nutrition, and appropriate fluid replacement is recommended for patients with mild COVID-19. Clinical sites can follow local guidelines and protocols in their countries and regions. In general, SOC will not differ between the two groups.
Prohibited medication
To ensure the clinical efficacy and safety of LHQW in the treatment of adult patients with mild and moderate COVID-19 and avoid the effect of drug interference and drug interaction, the following drugs are prohibited during the study (from enrollment/pre-dose to the end of the study).
- Hydroxychloroquine and lopinavir/ritonavir, remdesivir, systemic glucocorticoids, and antibodies against cytokines already approved for COVID-19;
- (Ii) Chinese herbal preparations or proprietary Chinese medicines (herbs and botanicals) that contain the same ingredients or have similar efficacy to LHQW.
Allowed medication
Any combination treatment and conventional drugs used for the treatment of COVID-19 during the study period must be recorded on the case report form. Symptomatic treatment, such as administration of antipyretic analgesics, adequate nutrition, and appropriate rehydration, in patients with mild COVID-19, is allowed by the protocol. Antipyretic and analgesic acetaminophen can be given as symptomatic treatment when the body temperature is ≥38.5 °C.
Outcome measurements
Efficacy evaluation
The primary efficacy endpoint is the median time to sustained improvement or resolution of the nine major symptoms during the 14-day observation period. The time to sustained improvement or the resolution of symptoms is defined as when the primary symptoms, including respiratory symptoms [stuffy or runny nose, sore throat, cough, shortness of breath (difficulty breathing)] and systemic symptoms (low energy or tiredness, muscle or body aches, headache, chills or shivering, feeling hot or feverish), are mild or absent (score is 1 or 0) for at least 24 hours. The COVID-19 symptom assessment scale is shown in Figure 2, refer to the guidelines of the U.S. Food and Drug Administration (FDA) (14).
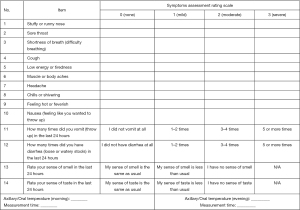
Secondary efficacy endpoints included the following indicators within the 14-day observation period:
- Proportion of patients with sustained improvement or resolution of the nine major symptoms.
- Median time to sustained improvement or resolution for each of the 9 primary symptoms.
- Median time to onset of antipyretic effect and cover to normal body temperature, which is defined as axillary temperature ≤37.0 °C or oral temperature ≤37.3 °C for at least 24 hours.
- Median time to sustained improvement or resolution of gastrointestinal symptoms.
- Median time to sustained improvement or resolution of smell loss and taste loss.
- Proportion of patients with all clinical symptoms improved or resolved.
- Time for SARS-CoV-2 virus tests to turn negative, and virus nucleic acid negative rate.
- Imaging (CT/chest X-ray) improvement rate. This endpoint will be assessed on a condition-specific center basis.
- Incidence of COVID-19-related severe/critical disease. Definition of severe COVID-19: clinical signs indicative of pneumonia (fever, cough, difficulty breathing, shortness of breath), and one of the following signs: respiratory rate >30 breaths per minute, severe dyspnea, or SpO2 <90% on room air. Critical COVID-19 means acute respiratory distress syndrome requiring mechanical ventilation, sepsis, infectious shock, or other organ failure requiring intensive care unit (ICU) care.
- COVID-19-related and all-cause mortality.
- Changes in infection-related inflammatory factors (white blood cell count, lymphocyte count, C-reactive protein, D-dimer). IL-6 and D-dimer tests will be performed 8 times in addition to the indicators mentioned above in Vietnam (Table 1).
Safety evaluation
Safety endpoints will be evaluated from the first dose to the end of follow-up, including:
- Vital signs (body temperature, heart rate, blood pressure, respiration), physical examination.
- Changes in laboratory tests: blood cell analysis (red blood cell count, hemoglobin, platelets, etc.), routine urine chemical analysis, liver and kidney function (glutamate transaminase, glutamic oxalacetic transaminase, total bilirubin, creatinine, urea nitrogen), cardiac enzymes (lactate dehydrogenase, creatine kinase, creatine kinase isoenzyme);
- Electrocardiogram;
- Adverse event (AE) rate and serious AE rate. If the conditions become worse, regular examinations should be performed until the test items are normal or return to baseline level or the investigator considers that follow-up is not necessary.
Costs/economic evaluation
Economic endpoints will be evaluated in the following three aspects:
- Health care cost. Cost analysis will be conducted from a societal perspective. The health care cost and the loss of human capital of enrolled patients will be systematically collected. (i) Direct costs include medical costs due to health care use and nonmedical personal and household costs due to COVID-19. Direct health care costs are divided into outpatient and inpatient service costs. Outpatient medical costs include registration, medical service, diagnosis and treatment, tests, examinations, prescription drugs, etc. Inpatient medical costs include daily hospitalization, treatment, examination, prescription drug, operations, tests, and other materials. Direct nonmedical costs include quarantine costs caused by the treatment of COVID-19 and its complications, as well as expenses incurred from quarantine policies, transportation, nutrition, and healthcare products. (ii) Indirect costs include the loss of productivity caused by the absence of work and premature death of the patient and his or her family members due to COVID-19 and its complications. They are estimated based on the labor cost of the patient and his or her family members.
- Health utility. Quality of life (QoL) is assessed using the 5-dimensional questionnaire by the EuroQol group (EQ-5D). QoL outcomes are evaluated in terms of quality-adjusted life-years (QALY) during the 14-day treatment period and follow-up period.
- Incremental cost-effectiveness ratio (ICER). Incremental cost-effectiveness analysis is the comparison of cost and effectiveness between the intervention group and the control group. ΔC/ΔE is known as the ICER, the ratio of the difference in total costs and the difference in effectiveness between the two groups.
Evaluation of AEs
All AEs are judged for their character, severity, and potential relationship to the investigational drug by authorized clinicians. The relationship between AE and the investigational drug is divided into five levels: definite, probable, possible, possibly unrelated, and definitely unrelated.
Data management
The case report form (CRF) will be completed by clinical investigators and submitted to the Data Management Unit [Blue Balloon (Beijing) Medical Research Co., Ltd.]. The database is established by the data manager before the first CRF is delivered. The data manager writes the data verification plan and verification procedures according to the range and interrelationship of the values of the endpoints in the CRF. All errors and corrections should be recorded in detail and kept in a secure location. After completing the above procedure, the principal investigator, trial statistics professional, and data manager will discuss the determination of the analysis set to which each case belongs, the handling of missing values, and the identification of outliers. After reviewing and discussing that the database created is correct, the database will be locked.
Statistical analysis
Sample size
The primary endpoint is the time (days) to sustained improvement or resolution of the 9 main clinical symptoms caused by COVID-19. Based on the results of a previous study that showed LHQW can shorten the symptom remission time by 3 days compared with the control group (8), it is assumed that the median time to sustained improvement or resolution in the control group is 12 days and that in the LHQW group is 9 days. Assuming that the total trial period is 1 year, the study will require 652 cases randomized in a 1:1 ratio to either the LHQW or placebo group for at least 95% power based on a 2-sided significance level of 0.05 (α=0.05, β=0.05). The calculations are performed using the Power Analysis and Sample Size (PASS) software. The enrollment is partly based on the SARS-CoV-2 antigen rapid test results. Assuming that 95% of cases based on a positive antigen rapid test will exhibit a positive result on a SARS-CoV-2 nucleic acid amplification test, the sample size is increased to 344 cases per group. The study will require approximately 860 eligible patients assuming a 20% dropout rate per group.
Randomization and blinding
Randomization numbers are generated using the Statistical Analysis System (SAS) statistical software package (SAS Inc., Cary, USA). A computer-generated 1:1 block randomization scheme is used to assign patients to either the LHQW group or the control group. Each consecutively coded subject is randomly enrolled by the sub-investigators in ascending order until the total number of cases allocated to the site is reached. Competitive recruitment is adopted for subject enrollment during the trial.
The dosage form and the dosage of LHQW and placebo are identical, packaged uniformly, and the same drug label is used. The drugs are randomly coded according to the randomization scheme, and the code is the unique identification number of the subject. Normally, investigators, subjects, inspectors, and data entry person are all be blinded. However, in emergency situations (such as serious adverse events, serious complications, etc.), when rescue measures need to be taken, the blinding can be urgently unblinding under the identification and signature of the person in charge. Then, notify the sponsor and the study leader within 24 hours of the emergency blinding and explain the reason.
Clinical data analyses and outputs
Analyses will be performed using the SAS Version 9.4 or higher. All statistical tests are two-tailed. If the P value is less than or equal to 0.05, the tested difference is considered statistically significant. Details regarding the statistical methods are as follows.
- Study populations. (i) Full analysis set (FAS): all subjects who are randomized and have taken at least one dose of study medication, according to the intention-to-treat principle. (ii) Per protocol set (PPS): all subjects who fully comply with the protocol. Compliance includes the treatment received, the availability of primary endpoint indicators, no major violation of the trial protocol, etc. PPS analysis is used for the primary efficacy indicators. (iii) Safety set (SS): all subjects who are randomized and have taken at least one dose of study medication and have at least one safety follow-up assessment.
- Primary and secondary efficacy endpoints. Based on FAS and PPS, the primary efficacy endpoint will be analyzed by the Kaplan-Meier method. The censored percentage, quartile, and 95% confidence interval will be listed, and a Kaplan-Meier curve will be generated. Comparisons between groups will be made using the log-rank test. For secondary efficacy endpoints, analysis will be made based on FAS, and different methods will be adopted according to different indicators. (i) The median time to sustained alleviation of a single symptom (any one of the nine main symptoms), the alleviation of fever, the alleviation of digestive symptoms, the alleviation of decreased sense of smell and taste, and the duration of viral shedding will be assessed using the same statistical methods as for the primary efficacy endpoint. (ii) The proportion of patients with alleviation of the nine main symptoms and patients with alleviation of all clinical symptoms, the rate of reduction in viral shedding, the improvement rate of imaging (CT/chest X-ray), the incidence of severe and critical diseases related to COVID-19, and the incidence of death and all-cause death related to COVID-19 will be analyzed using the χ2 test or Fisher’s exact probability method to compare the differences between groups. (iii) Infection-related inflammatory indices compared to baseline will be compared between groups via a t-test.
- Analysis of safety. All AEs recorded on the eCRF will be coded using the Medical Dictionary for Regulatory Activities (MedDRA) dictionary. All AE summaries will be restricted to treatment-emergent adverse events (TEAEs). Summaries of the incidence of TEAEs, drug-related TEAEs, serious adverse events (SAEs), and TEAEs leading to discontinuation of the study will be presented. The number, frequency, and incidence of TEAEs, drug-related TEAEs, SAEs, and TEAEs leading to discontinuation of the study by system organ class, the preferred term (PT), and severity will be presented. A cross-tabulation of laboratory tests, electrocardiograms, and physical examinations before and after medication will be listed. Any abnormality will be listed and explained. Data on vital signs and changes from baseline will be summarized with descriptive statistics.
- Economic analysis. Economics analysis will be carried out in the form of a cost-effectiveness analysis (CEA), cost-utility analysis (CUA), and sensitivity analysis. (i) CEA: the primary efficacy endpoints will be compared between the LHQW group and the placebo group. The ratio of the difference in costs and the difference in efficacy between the two groups will be calculated. (ii) CUA: the incremental cost-to-utility ratio ΔC/ΔU will be compared between the intervention group and control group, the QALY indicator using the patient’s individual data will be assessed, and a Markov model will be used to model the lifetime period of CUA. (iii) Sensitivity analysis: a one-way sensitivity analysis and probabilistic sensitivity analysis on cost, effect, and utility indicators will be performed. The one-way sensitivity analysis will be implemented using the extreme analysis model with the upper and lower limits of the 95% confidence interval. A probabilistic sensitivity analysis will also be carried out using Monte Carlo simulation. The nonparametric bootstrap method will be used to analyze sampling uncertainty.
- Exploratory analysis: exploratory analysis will be carried out for subgroups with important factors, such as gene sequence analysis (variant strains) and imaging (CT or chest X-ray).
- Analysis of demographic and baseline data. Continuous data will be summarized in terms of the mean ± SD, and median (quartile, or minimum and maximum value). Categorical data will be summarized with counts and percentages. The inferential statistical results (P values) will be listed with the descriptive results.
- Analysis of medication compliance and concomitant medications. The percentage of subjects with medication compliance in the range of 80–120% will be calculated and compared using the χ2 test or Fisher’s exact probability method to assess the differences between groups. The actual dose and the time of drug exposure will be compared using a t-test. The concomitant medications will be coded by WHO anatomical therapeutic chemical (ATC) and summarized according to the ATC classification. The number and rate of concomitant medications will be calculated.
Quality control
Staff training
To ensure the safety of the subjects and the strict implementation of the trial, all personnel directly involved in the study shall receive the necessary training and obtain the relevant certification, such as GCP training certificates. In addition, the following specific training contents are needed: (I) a kick-off meeting and trial program training meeting concerning the study protocol and procedures; (II) webinars about relevant standard operating procedures (such as nasopharyngeal swab sampling, etc.); and (III) relevant videos provided by the study center. All investigators will be required to submit signatures to certify the completion of the required training tasks before the study is officially carried out. The purpose of training is to ensure that investigators have a full understanding of the specific connotation of each indicator, can describe subjective symptoms objectively, without induction or prompting, and conduct the inspections according to the time point and method specified in the protocol.
Monitoring
The sponsor and participating research centers will establish quality control and quality assurance systems, respectively. The sponsor will engage a third-party company as the contract research organization (CRO) for this trial to ensure that a qualified clinical research associate (CRA) is responsible for overseeing the trial and ensuring that the study complies with the approved protocol, GCP, and other relevant regulations. Before the start of the study, the project manager will cooperate with the quality control department to formulate and complete the clinical management plan (CMP) according to this trial protocol. CMP describes in detail specific supervisory performers of the inspections, the frequency of inspections, the extent of supervisory implementation, and the distribution of supervisory reports. Detailed requirements for monitoring the research centers will also be recorded in the CMP. The CRA will perform the inspection tasks according to the CMP and the actual study progress and submit the inspection report and follow-up letter after the monitoring visit promptly.
Safety and ethics
The LHQW used in this trial has been approved for clinical use. To ensure that patients are treated effectively during the study, all subjects will receive SOC. Patients will be informed of the objectives, methods, and procedures of the trial before enrollment. Investigators will also answer any questions from the subjects in detail and will not perform this trial until written informed consent is obtained. The participating units must comply with good clinical practice (GCP) regulations and clinical trial protocols and be subject to review by relevant departments.
The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the study protocol is established by the institutional review boards at the relevant study sites and has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2022.24). All subcenters must submit ethics applications to their centers using the ethics of the lead institution. Informed consent will be obtained from all subjects and/or their legal guardians.
Registration details and study timeline
This study was registered at the Chinese Clinical Trials Registry (registration No. ChiCTR2200056727) with the public title of “A Randomized, Double-Blind, International Multi-Center Clinical Study to Investigate Efficacy and Safety of Lianhua Qingwen Capsules Compared to Placebo and Combined with Standard of Care (SOC) in Adult Patients with Mild to Moderate COVID-19”. The first informed consent was signed on February 28, 2022; Subject enrollment was completed on November 31, 2022; The last subject visit was completed on December 15, 2022; Data collation was completed on January 28, 2023 and the database lock was completed on February 7, 2023.
Dissemination policy
The trial will be disseminated at regional and national academic conferences. All data and analyses will remain blinded until the main results are released. Finally, the main results will be published in a peer-reviewed journal (according to the journal’s guidelines). In addition, a lay summary of the study results will be circulated to potentially interested parties.
Discussion
Currently, the global COVID-19 epidemic has shown a new pattern with the previously predominant delta strain replaced by the novel variant omicron which adds to more powerful, faster, and stealthier transmission of the disease (15). The existing prevention and control strategies, including public health measures (such as wearing masks, health checks at airports, and border crossings), social distancing, and even short-term quarantine, are becoming increasingly difficult to maintain. Even in China, where epidemic prevention and control measures are stricter, omicron-induced local outbreaks often occur (in Shanghai, for example, the existing confirmed cases exceeded the cases of the Wuhan outbreak two years ago). Moreover, the omicron strain generally has a strong tendency to evade immunity induced by vaccines (16-19), and it is difficult to avoid reinfection even for people who have received booster shots or recovered from COVID-19 (20-22). As a result, the immune protective effect of vaccination has been greatly weakened, and the current plan of opening the country to normal economic and social life through vaccination policy has been greatly challenged. Facing severe epidemic pressure, the research and development of effective and accessible drugs are particularly important and urgent.
Although several neutralizing antibodies have been authorized for emergency use [such as sotrovimab by GlaxoSmithKline and Vir Biotechnology, AZD7442 (AZD8895/AZD1061) by AstraZeneca, etc.], disadvantages of these treatments include the possibility of exacerbating the disease, triggering cytokine storms and ineffectiveness against mutant strains (23-25). At the same time, the cost of neutralizing antibodies increases the economic burden of treatment. Currently, small-molecule drugs are the focus of anti-COVID-19 drug research and development, with advantages such as ease of production, ease of preservation, and suitability for the treatment of broad-spectrum and multivariant viruses. However, small molecule drugs have unavoidable disadvantages, such as a narrow drug prescribing time window, a single target, and easy to have a drug resistance (26-29). Although small-molecule drugs such as molnupiravir and paxlovid have been approved for the clinical treatment of COVID-19, their efficacy remains to be confirmed. Moreover, in the current outbreak caused by the omicron strain, the symptoms of infected people are generally mild, and many are even mostly asymptomatic. However, these drugs are not inexpensive, for example, Paxlovid cost $530 per course (three tablets each time, twice daily for five days, in other words, it’s equivalent to $17.67 per tablet). Therefore, from an economic point of view, this price greatly reduces the accessibility and affordability of small-molecule drugs, and more importantly, it may be unnecessary to apply to most infected people at the present stage. Traditional Chinese Medicine (TCM) has been used for thousands of years in China, with the advantages of a simple source, low cost, multiple targets, and preservation at room temperature. The report of the WHO Expert Meeting on the Evaluation of Traditional Chinese Medicine in the Treatment of COVID-19 has clearly affirmed the safety and efficacy of TCM in treating COVID-19, and encouraged its member states to consider the possibility of applying TCM within their healthcare system and regulatory framework (30). LHQW is a proven effective Chinese patent medicine with good clinical application prospects.
According to the guidance of the collateral disease theory, LHQW was originally developed due to the SARS outbreak and played an active role in the fight against influenza A (H1N1) and COVID-19 in China. However, against the backdrop of the current global epidemic that is still growing and the urgent need to develop COVID-19 treatment drugs, the consideration should be whether the drugs have actual therapeutic efficacy and good safety after rigorous clinical research and whether they can be quickly applied to a large number of people. Therefore, this trial has important clinical value. First, LHQW in capsule dosage form, the same dosage form as chemical medicine, is convenient for clinical application. Second, LHQW has the fingerprint of the main medicinal ingredients, which ensures the stability of drug quality and facilitates later clinical promotion and application (31,32). Third, previous studies had confirmed the clinical benefits and high safety of LHQW in the treatment of COVID-19 (8-10,33,34). However, these studies were all conducted in China with small samples and had certain limitations. Considering the differences among different regions and populations, high-level evidence-based medical data will be collected in the international multicenter RCT study. Combined with the current general trend of COVID-19 clinical characteristics and the therapeutic advantages of LHQW, this trial will focus more on the evaluation of clinical symptoms compared with previous studies. Firstly, we have standardized the COVID-19 symptom evaluation scale based on the U.S. FDA guidelines (14). Secondly, we will comprehensively and systematically evaluate the effects of LHQW on COVID-19 symptoms, including respiratory symptoms, gastrointestinal symptoms, systemic symptoms, smell, and taste, rather than just evaluating the three symptoms of fever, fatigue, and cough. Furthermore, we will take the median time to sustained improvement or resolution of the nine major symptoms as the primary efficacy endpoint, and pay more attention to the median time to onset of antipyretic effect and cover to normal body temperature. From this, more detailed evidence will be provided to prove the efficacy of LHQW in treating COVID-19. However, under the China’s ‘zero COVID-19’ policy, we will carry out the evaluation of inflammation indicators but not explore additional hospitalization indicators. We will also develop relevant economic endpoints, including utility, cost, and ICER, to evaluate the possible economic benefits brought by LHQW treatment and provide evidence for the accessibility and affordability of LHQW.
This is the first international multicenter RCT trial of Chinese patent medicine for the treatment of early COVID-19 in accordance with WHO guidelines on COVID-19 management. We expect this clinical study to provide higher-level evidence for the efficacy, safety and economy of LHQW in the treatment of COVID-19. On the one hand, effective and affordable treatments and drugs can be provided for COVID-19 patients, especially in countries where TCM already has a basis. On the other hand, this study may focus researchers’ attention on the treatment of COVID-19 by TCM, which is increasingly popular in western countries.
Acknowledgments
The authors wish to acknowledge Youping Li, professor of West China Hospital of Sichuan University, Yuanlin Song, professor of Zhongshan Hospital Fudan University, and Zhenhua Jia, professor of National Key Laboratory of Collateral Disease Research and Innovative Chinese Medicine, for valuable discussions of the trial. We also thank Dr. Zhang Ling, Hebei Chest Hospital, Dr. Wu Lei, Hebei Provincial Hospital of Traditional Chinese Medicine, and Dr. Jiang Rongmeng, Beijing Ditan Hospital Capital Medical University, for their constructive suggestions for clinical implementation.
Funding: This trial is financially supported by Shijiazhuang Yiling Pharmaceutical Co. Ltd. The funder’s primary purpose is to provide funding and interventional drugs and is not involved in this trial, including the planning, execution, data management, statistical analysis, evaluation, or writing of the study. The execution and design of the study are entrusted to third-party teams with no relevant interests. This work is also supported by National Administration of Traditional Chinese Medicine (No. ZYYCXTU-D-202206).
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-281/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-281/coif). NSZ serves as Editor-in-Chief of Journal of Thoracic Disease. All authors report that this trial is financially supported by Shijiazhuang Yiling Pharmaceutical Co. Ltd. NSZ, ZFY, and JPZ also report funding from National Administration of Traditional Chinese Medicine (No. ZYYCXTU-D-202206). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the study protocol is established by the institutional review boards at the relevant study sites and has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2022.24). All subcenters must submit ethics applications to their centers using the ethics of the lead institution. Informed consent will be obtained from all subjects and/or their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- WHO. Coronavirus disease (COVID-19) pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409-24. [Crossref] [PubMed]
- Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol 2022;94:1738-44. [Crossref] [PubMed]
- Yu W, Guo Y, Zhang S, et al. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: A systematic review and analysis. J Med Virol 2022;94:5790-801. [Crossref] [PubMed]
- Practice, B.B. Coronavirus disease 2019 (COVID-19). 2023. Available online: https://bestpractice.bmj.com/topics/zh-cn/3000201/management-recommendations.
- Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020;156:104761. [Crossref] [PubMed]
- Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 2021;85:153242. [Crossref] [PubMed]
- Shi C, Wu M, Yang K, et al. Lianhua Qingwen Capsules Reduced the Rate of Severity in Patients with COVID-19: A System Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med 2022;2022:9617429. [Crossref] [PubMed]
- Wang DC, Yu M, Xie WX, et al. Meta-analysis on the effect of combining Lianhua Qingwen with Western medicine to treat coronavirus disease 2019. J Integr Med 2022;20:26-33. [Crossref] [PubMed]
- Gong X, Yuan B, Yuan Y, et al. Efficacy and Safety of Lianhuaqingwen Capsules for the Prevention of Coronavirus Disease 2019: A Prospective Open-Label Controlled Trial. Evid Based Complement Alternat Med 2021;2021:7962630. [Crossref] [PubMed]
- Organization, W.H. COVID-19 clinical management: living guidance, 25 January 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/338882/WHO-2019-nCoV-clinical-2021.1-eng.pdf
- Commission, T.H. Scheme for Diagnosis and Treatment of 2019 Novel Coronavirus Pneumonia (The 8th Trial Edition). 2021. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a/files/a449a3e2e2c94d9a856d5faea2ff0f94.pdf
- FDA, C., CBER. Assessing COVID-19-Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment. Guidance for Industry. Sept, 2020. Accessed on August 25, 2022. Available online: https://www.fda.gov/media/142143/download.
- Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022;603:715-20. [Crossref] [PubMed]
- Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022;602:657-63. [Crossref] [PubMed]
- Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022;602:676-81. [Crossref] [PubMed]
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022;602:671-5. [Crossref] [PubMed]
- Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654-6. [Crossref] [PubMed]
- Naito T, Yan Y, Tabe Y, et al. Real-world evidence for the effectiveness and breakthrough of BNT162b2 mRNA COVID-19 vaccine at a medical center in Japan. Hum Vaccin Immunother 2022;18:1-2. [Crossref] [PubMed]
- Chandan S, Khan SR, Deliwala S, et al. Postvaccination SARS-CoV-2 infection among healthcare workers: A systematic review and meta-analysis. J Med Virol 2022;94:1428-41. [Crossref] [PubMed]
- Vaishya R, Sibal A, Malani A, et al. Symptomatic post-vaccination SARS-CoV-2 infections in healthcare workers- A multicenter cohort study. Diabetes Metab Syndr 2021;15:102306. [Crossref] [PubMed]
- Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 2020;577:399-404. [Crossref] [PubMed]
- Winarski KL, Tang J, Klenow L, et al. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc Natl Acad Sci U S A 2019;116:15194-9. [Crossref] [PubMed]
- Administration, F.a.D. FDA updates Sotrovimab emergency use authorization. 2022. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization
- Shinkai M, Tsushima K, Tanaka S, et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect Dis Ther 2021;10:2489-509. [Crossref] [PubMed]
- Fujii S, Ibe Y, Ishigo T, et al. Early favipiravir treatment was associated with early defervescence in non-severe COVID-19 patients. J Infect Chemother 2021;27:1051-7. [Crossref] [PubMed]
- Bragstad K, Hungnes O, Litleskare I, et al. Community spread and late season increased incidence of oseltamivir-resistant influenza A(H1N1) viruses in Norway 2016. Influenza Other Respir Viruses 2019;13:372-81. [Crossref] [PubMed]
- Tang JW, Kennedy M, Lackenby A, et al. Transmitted and acquired oseltamivir resistance during the 2018-2019 influenza season. J Infect 2019;79:612-25. [Crossref] [PubMed]
-
WHO Expert Meeting on Evaluation of Traditional Chinese Medicine in the Treatment of COVID-19 2022 . - Jia W, Wang C, Wang Y, et al. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. ScientificWorldJournal 2015;2015:731765. [Crossref] [PubMed]
- Zhou Y, Niu M, Zhang D, et al. Screening for Anti-Inflammation Quality Markers of Lianhua Qingwen Capsule Based on Network Pharmacology, UPLC, and Biological Activity. Front Pharmacol 2021;12:648439. [Crossref] [PubMed]
- Fan SJ, Liao JK, Wei L, et al. Treatment efficacy of Lianhua Qingwen capsules for eraly-stage COVID-19. Am J Transl Res 2022;14:1332-8. [PubMed]
- Hu C, He B, Gong F, et al. The Adverse Reactions of Lianhua Qingwen Capsule/Granule Compared With Conventional Drug in Clinical Application: A Meta-Analysis. Front Pharmacol 2022;13:764774. [Crossref] [PubMed]


